Abstract
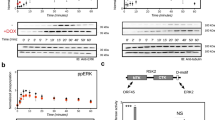
HUMAN cytomegalovirus (HCMV) is a major pathogen in immunosuppressed individuals, including patients with acquired immune deficiency syndrome. The nucleoside analogue ganciclovir (9-(l,3-dihydroxy-2-propoxymethyl)-guanine) is one of the few drugs available to treat HCMV infections, but resistant virus is a growing problem in the clinic1 and there is a critical need for new drugs. The study of ganciclovir-resistant mutants has indicated that the selective action of ganciclovir depends largely on virus-controlled phosphorylation in HCMV-infected cells2–5. The enzyme(s) responsible have not been identified. Here we report that the HCMV gene UL97, whose predicted product shares regions of homology with protein kinases, guanylyl cyclase and bacterial phosphotransferases6–8, controls phosphorylation of ganciclovir in HCMV-infected cells. A four-amino-acid deletion of UL97 in a conserved region, which in cyclic AMP-dependent protein kinase participates in substrate recognition9, causes impaired ganciclovir phosphorylation. The implications of these results for antiviral drug development and drug resistance are discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Drew, W. L. et al. J. infect Dis. 163, 716–719 (1991).
Biron, K. K. et al. Proc. natn. Acad. Sci. U.S.A. 82, 2473–2477 (1985).
Freitas, V. R., Smee, D. F., Chernow, M., Boehme, R. & Matthews, T. R. Antimicrob. Ag. Chemother. 28, 240–245 (1985).
Biron, K. K. et al. Proc. natn. Acad. Sci. U.S.A. 83, 8769–8773 (1986).
Stanat, S. C. et al. Antimicrob. Ag. Chemother. 35, 2191–2197 (1991).
Chee, M. S., Lawrence, G. L. & Barrell, B. G. J. gen. Virol. 70, 1151–1160 (1989).
Brenner, S. Nature 329, 21 (1987).
Singh, S. et al. Nature 334, 708–712 (1988).
Knighton, D. R. et al. Science 253, 414–420 (1991).
McGeoch, D. J. et al. J. gen. Virol. 69, 1531–1574 (1988).
Davison, A. J. & Scott, J. E. J. gen. Virol. 67, 1759–1816 (1986).
Baer, R. et al. Nature 310, 207–211 (1984).
Smith, R. F. & Smith, T. F. J. Virol. 63, 450–455 (1989).
Hanks, S. K., Quinn, A. M. & Hunter, T. Science 241, 42–52 (1988).
Agut, H. et al. Res. Virol. 140, 219–228 (1989).
Di Luca, D., Katsafanas, G., Schrimer, E. C., Balachandran, N. & Frenkel, N. Virology 175, 199–210 (1990).
Fyfe, J. A., Keller, P. M., Furman, P. A., Miller, R. L. & Elion, G. B. J. biol Chem. 253, 8721–8727 (1978).
Chee, M. S. et al. Curr. Top. Microbiol. Immun. 154, 125–169 (1990).
Mar, E.-C., Patel, P. C. & Huang, E.-S. J. gen. Virol. 57, 149–156 (1981).
Michelson, S., Tardy-Panit, M. & Barzo, O. Virology 134, 259–268 (1984).
Roby, C. & Gibson, W. J. Virol. 59, 714–727 (1986).
Britt, W. J. & Auger, D. J. Virol. 59, 185–188 (1986).
Somogyi, T., Michelson, S. & Masse, M.-J., O. Virology 174, 276–285 (1990).
Littler, E., Stuart, A. D. & Chee, M. S. Nature 358, 160–162 (1992).
Lau, Y.-F. & Kan, Y. W. Proc. natn. Acad. Sci. U.S.A. 80, 5225–5229 (1983).
Fleckenstein, B., Muller, I. & Collins, J. Gene 18, 39–46 (1982).
Fyfe, J. A., McKee, S. A. & Keller, P. M. Molec. Pharmac. 24, 316–323 (1983).
Shoji, S. et al. Proc. natn. Acad. Sci. U.S.A. 78, 848–851 (1981).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sullivan, V., Talarico, C., Stanat, S. et al. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature 358, 162–164 (1992). https://doi.org/10.1038/358162a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/358162a0
This article is cited by
-
Serum SAA1 and APOE are novel indicators for human cytomegalovirus infection
Scientific Reports (2017)
-
Liquid chromatography–mass spectrometry methods for the intracellular determination of drugs and their metabolites: a focus on antiviral drugs
Analytical and Bioanalytical Chemistry (2017)
-
Liquid chromatography tandem mass spectrometry quantitation of intracellular concentrations of ganciclovir and its phosphorylated forms
Analytical and Bioanalytical Chemistry (2015)
-
The interplay between host and viral factors in shaping the outcome of cytomegalovirus infection
Immunology & Cell Biology (2007)