Abstract
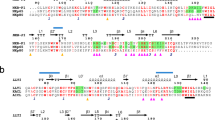
Natural killer (NK) cells express cell-surface receptors of the immunoglobulin and C-type lectin superfamilies that recognize major histocompatibility complex (MHC) class I peptides and inhibit NK-cell-mediated cytotoxicity1. These inhibitory receptors possess ITIM sequences (for immunoreceptor tyrosine-based inhibitory motifs) in their cytoplasmic domains that recruit SH2-domain-containing protein tyrosine phosphatases, resulting in inactivation of NK cells2,3,4. Certain isoforms of these NK-cell receptors lack ITIM sequences and it has been proposed that these ‘non-inhibitory’ receptors may activate, rather than inhibit, NK cells4,5,6. Here we show that DAP12, a disulphide-bonded homodimer containing an immunoreceptor tyrosine-based activation motif (ITAM) in its cytoplasmic domain, non-covalently associates with membrane glycoproteins of the killer-cell inhibitory receptor (KIR) family without an ITIM in their cytoplasmic domain. Crosslinking of KIR–DAP12 complexes results in cellular activation, as demonstrated by tyrosine phosphorylation of cellular proteins and upregulation of early-activation antigens. Phosphorylated DAP12 peptides bind ZAP-70 and Syk protein tyrosine kinases, suggesting that the activation pathway is similar to that of the T- and B-cell antigen receptors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lanier, L. L. NK cells: from no receptors to too many. Immunity 6, 371–378 (1997).
Burshtyn, D. N. et al. Recruitment of tyrosine phosphatase HCP by the killer cell inhibitory receptor. Immunity 4, 77–85 (1996).
Olcese, L. et al. Human and mouse killer-cell inhibitory receptors recruit PTP1C and PTP1D protein tyrosine phosphatases. J. Immunol. 156, 4531–4534 (1996)
Houchins, J. P., Lanier, L. L., Niemi, E., Phillips, J. H. & Ryan, J. C. Natural killer cell cytolytic activity is inhibited by NKG2-A and activated by NKG2-C. J. Immunol. 158, 3603–3609 (1997).
Biassoni, R. et al. The human leukocyte antigen (HLA)-C-specific ‘activatory’ or ‘inhibitory’ natural killer cell receptors display highly homologous extracellular domains but differ in their transmembrane and intracytoplasmic portions. J. Exp. Med. 183, 645–650 (1996).
Mason, L. H. et al. The Ly-49D receptor activates murine natural killer cells. J. Exp. Med. 184, 2119–2128 (1996).
Olcese, L. et al. Human killer cell activatory receptors for MHC class I molecules are included in a multimeric complex expressed by Natural Killer cells. J. Immunol. 158, 5083–5086 (1997).
Reth, M. Antigen receptor tail clue. Nature 338, 383–384 (1989).
Chan, A. C., Desai, D. M. & Weiss, A. The role of protein tyrosine kinases and protein tyrosine phosphatases in T cell antigen receptor signal transduction. Annu. Rev. Immunol. 12, 555–592 (1994).
Baker, E., D'Andrea, A., Phillips, J. H., Sutherland, G. R. & Lanier, L. L. Natural killer cell receptor for HLA-B allotypes, NKB1: map position 19q13.4. Chrom. Res. 3, 511 (1995).
Meyaard, L. et al. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity 7, 282–290 (1997).
Wagtmann, N., Rojo, S., Eichler, E., Mohrenweiser, H. & Long, E. O. Anew human gene complex encodes the killer cell inhibitory receptors and a related family of monocyte/macrophage receptors. Curr. Biol. 7, 615–618 (1997).
Chang, C. et al. Molecular characterization of human CD94: a type II membrane glycoprotein related to the C-type lectin superfamily. Eur. J. Immunol. 25, 2433–2437 (1995).
Houchins, J. P., Yabe, T., McSherry, C. & Bach, F. H. DNA sequence analysis of NKG2, a family of related cDNA clones encoding type II integral membrane proteins on human natural killer cells. J. Exp. Med. 173, 1017–1020 (1991).
Samaridis, J. & Colonna, M. Cloning of novel immunoglobulin superfamily receptors expressed on human myeloid and lymphoid cells: structural evidence for new stimulatory and inhibitory pathways. Eur. J. Immunol. 27, 660–665 (1997).
Colonna, M. & Samaridis, J. Cloning of Ig-superfamily members associated with HLA-C and HLA-B recognition by human NK cells. Science 268, 405–408 (1995).
Clevers, H., Alarcon, B., Wileman, T. & Terhorst, C. The T cell receptor/CD3 complex: a dynamic protein ensemble. Annu. Rev. Immunol. 6, 629–662 (1988).
Iwashima, M., Irving, B. A., van Oers, N. S. C., Chang, A. C. & Weiss, A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science 263, 1136–1139 (1994).
Chan, A. C. et al. Differential expression of ZAP-70 and Syk protein tyrosine kinases, and the role of this family of protein tyrosine kinases in TcR signaling. J. Immunol. 152, 4758–4766 (1994).
Bléry, M. et al. Reconstituted killer cell inhibitory receptors for major histocompatibility complex class I molecules control mast cell activation induced via immunoreceptor tyrosine-based activation motifs. J. Biol. Chem. 272, 8989–8996 (1997).
Hayami, K. et al. Molecular cloning of a novel murine cell-surface glycoprotein homologous to killer cell inhibitory receptors. J. Biol. Chem. 272, 7320–7326 (1997).
Kubagawa, H., Burrows, P. D. & Cooper, M. D. Anovel pair of immunoglobulin-like receptors expressed by B cells and myeloid cells. Proc. Natl Acad. Sci. USA 94, 5261–5266 (1997).
Takase, K. et al. Anew 12-kilodalton dimer associated with pre-TCR complex and clonotype-independent CD3 complex on immature thymocytes. J. Immunol. 159, 741–747 (1997).
Onihsi, M. et al. Applications of retrovirus-mediated expression cloning. Exp. Hematol. 24, 324–329 (1996).
Wu, J., Katzav, S. & Weiss, A. Afunctional T-cell receptor signaling pathway is required for p95vav activity. Mol. Cell. Biol. 15, 4337–4346 (1995).
Lanier, L. L., Yu, G. & Phillips, J. H. Co-association of CD3ζ with a receptor (CD16) for IgG Fc on human natural killer cells. Nature 342, 803–805 (1989).
Phillips, J. H. et al. CD94 and a novel associated protein (94AP) form a NK cell receptor involved in the recognition of HLA-A, -B, and -C allotypes. Immunity 5, 163–172 (1996).
Lanier, L. L. & Recktenwald, D. J. Multicolor immunofluorescence and flow cytometry. Methods: A Companion to Methods in Enzymology 2, 192–199 (1991).
Acknowledgements
We thank F. Bazan and J. Bolen for helpful discussion, C. Saraiya for expert technical assistance, C. Turck and A. Weiss for phosphopeptides and antibodies, J. Ford, T. McClanahan and K.Bacon for cDNA libraries, J. A. Katheiser, G. Burget and M. Andonian for graphics, J. Cupp, E. Callas, E.Murphy and D. Polakoff for flow cytometry, D. Gorman for DNA sequencing and D. Liggett for oligonucleotides. DNAX Research Institute is supported by Schering Plough Corporation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lanier, L., Corliss, B., Wu, J. et al. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature 391, 703–707 (1998). https://doi.org/10.1038/35642
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35642
This article is cited by
-
Themis2 regulates natural killer cell memory function and formation
Nature Communications (2023)
-
CD8+ T cells maintain killing of MHC-I-negative tumor cells through the NKG2D–NKG2DL axis
Nature Cancer (2023)
-
Microglial TYROBP/DAP12 in Alzheimer’s disease: Transduction of physiological and pathological signals across TREM2
Molecular Neurodegeneration (2022)
-
Natural killer cells: a promising immunotherapy for cancer
Journal of Translational Medicine (2022)
-
Membrane protein trafficking in the anti-tumor immune response: work of endosomal-lysosomal system
Cancer Cell International (2022)