Abstract
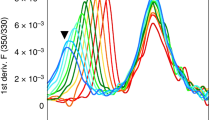
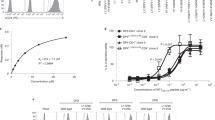
T LYMPHOCYTES recognize antigen-derived peptides associated with major histocompatibility complex (MHC) class I or class II proteins1,2. Peptide is critical in class I heavy-chain folding and/or stable association with β2-microglobulin3–6. Although data exist suggesting a relationship between class II structure and peptide association7–9, no equivalent positive contribution of peptide to the folding state or stability of class II dimers has yet been demonstrated. We report here that most purified EαkEβk molecules leaving low pH in the absence of specific peptide lack a compact, stable dimeric structure. Brief exposure to the appropriate peptide just before and during neutralization promotes this specific conformation in proportion to stably bound peptide, indicating that peptide is important in determining class II MHC structure. Our results also indicate that efficient generation of long-lived peptide-class II complexes involves two stages: initial peptide binding in an acidic environment, which enhances the ability of class II to enter a conformation, from which stabilization upon neutralization results in high-affinity binding of previously associated peptide.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Schwartz, R. H. A. Rev. Immun. 3, 237–261 (1985).
Townsend, A. & Bodmer, H. A. Rev. Immun. 7, 601–624 (1989).
Townsend, A. et al. Nature 340, 443–448 (1989).
Lie, W. R. et al. Nature 344, 439–441 (1990).
Townsend, A. et al. Cell 62, 285–295 (1990).
Schumacher, T. N. M. et al. Cell 62, 563–567 (1990).
Sadegh-Nasseri, S. & McConnell, H. M. Nature 337, 274–276 (1989).
Dornmair, K., Rothenhausler, B. & McConnell, H. M. Cold Spring Harb. Symp. quant. Biol. 54, 409–416 (1989).
Mellins, E. et al. Nature 343, 71–74 (1990).
Babbitt, B. P., Allen, P. M., Matsueda, E., Haber, E. & Unanue, E. R. Nature 317, 359–361 (1985).
Buus, S., Sette, A., Colon, S., Miles, G. & Grey, H. M. Science 235, 1353–1358 (1987).
Watts, T. H. & McConnell, H. M. Proc. natn. Acad. Sci. U.S.A. 83, 9660–9664 (1986).
Viguier, M., Dornmair, K., Clark, B. R. & McConnell, H. M. Proc. natn. Acad. Sci. U.S.A. 87, 7170–7174 (1990).
Jensen, P. E. J. exp. Med. 171, 1779–1784 (1990).
Harding, C. V., Roof, R. W., Allen, P. M. & Unanue, E. R. Proc. natn. Acad. Sci. U.S.A. 88, 7170–7174 (1991).
Buus, S., Sette, A., Colon, S. & Grey, H. M. Science 242, 1045–1047 (1988).
Buus, S., Sette, A., Colon, S. M., Jenis, D. M. & Grey, H. M. Cell 47, 1071–1077 (1986).
Rothenhausler, B., Dornmair, K. & McConneil, H. M. Proc. natn. Acad. Sci. U.S.A. 87, 352–354 (1990).
Germain, R. N. & Hendrix, L. R. Nature 353, 134–139 (1991).
Harding, C. V. & Unanue, E. R. Cell Regulation 1, 499–509 (1990).
Dornmair, K. & McConnell, H. M. Proc. natn. Acad. Sci. U.S.A. 87, 4134–4138 (1990).
Bevan, M. J. Nature 342, 478–479 (1989).
Ozato, K., Mayer, N. & Sachs, D. H. J. Immun. 124, 533–540 (1980).
Laemmli, U. K. Nature 227, 680–685 (1970).
Wray, W., Boulikas, T., Wray, V. P. & Hancock, R. Prac. Biochem 118, 197–202 (1981).
Busch, R., Strang, G., Howland, K. & Rothbard, J. B. Int. Immun. 2, 443–451 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sadegh-Nasseri, S., Germain, R. A role for peptide in determining MHC class II structure. Nature 353, 167–170 (1991). https://doi.org/10.1038/353167a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/353167a0