Abstract
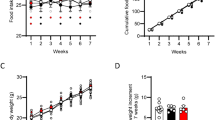
Oleylethanolamide (OEA) is a natural analogue of the endogenous cannabinoid anandamide. Like anandamide, OEA is produced in cells in a stimulus-dependent manner and is rapidly eliminated by enzymatic hydrolysis, suggesting a function in cellular signalling1. However, OEA does not activate cannabinoid receptors and its biological functions are still unknown2. Here we show that, in rats, food deprivation markedly reduces OEA biosynthesis in the small intestine. Administration of OEA causes a potent and persistent decrease in food intake and gain in body mass. This anorexic effect is behaviourally selective and is associated with the discrete activation of brain regions (the paraventricular hypothalamic nucleus and the nucleus of the solitary tract) involved in the control of satiety. OEA does not affect food intake when injected into the brain ventricles, and its anorexic actions are prevented when peripheral sensory fibres are removed by treatment with capsaicin. These results indicate that OEA is a lipid mediator involved in the peripheral regulation of feeding.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Di Marzo, V. et al. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 372, 686–691 (1994).
Piomelli, D., Beltramo, M., Giuffrida, A. & Stella, N. Endogenous cannabinoid signaling. Neurobiol. Dis. 5, 462–473 (1998).
Bachur, N. R., Masek, K., Melmon, K. L. & Udenfriend, S. Fatty acid amides of ethanolamine in mammalian tissues. J. Biol. Chem. 240, 1019–1024 (1965).
Schmid, H. H., Schmid, P. C. & Natarajan, V. The N-acylation-phosphodiesterase pathway and cell signalling. Chem. Phys. Lipids 80, 133–142 (1996).
Chapman, K. D. Emerging physiological roles for N-acylphosphatidylethanolamine metabolism in plants: signal transduction and membrane protection. Chem. Phys. Lipids 108, 221–229 (2000).
Giuffrida, A. et al. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nature Neurosci. 2, 358–363 (1999).
Berdyshev, E. V., Schmid, P. C., Dong, Z. & Schmid, H. H. Stress-induced generation of N-acylethanolamines in mouse epidermal JB6 P+ cells. Biochem. J. 346, 369–374 (2000).
Cadas, H., Gaillet, S., Beltramo, M., Venance, L. & Piomelli, D. Biosynthesis of an endogenous cannabinoid precursor in neurons and its control by calcium and cAMP. J. Neurosci. 16, 3934–3942 (1996).
Cadas, H., di Tomaso, E. & Piomelli, D. Occurrence and biosynthesis of endogenous cannabinoid precursor, N-arachidonoyl phosphatidylethanolamine, in rat brain. J. Neurosci. 17, 1226–1242 (1997).
Beltramo, M. et al. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science 277, 1094–1097 (1997).
Schmid, P. C., Zuzarte-Augustin, M. L. & Schmid, H. H. Properties of rat liver N-acylethanolamine amidohydrolase. J. Biol. Chem. 260, 14145–14149 (1985).
Cravatt, B. F. et al. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384, 83–87 (1996).
Devane, W. A. et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258, 1946–1949 (1992).
Williams, C. M. & Kirkham, T. C. Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology 143, 315–317 (1999).
Khanolkar, A. D. & Makriyannis, A. Structure–activity relationships of anandamide, an endogenous cannabinoid ligand. Life Sci. 65, 607–616 (1999).
Pertwee, R. G. Cannabinoid receptor ligands: clinical and neuropharmacological considerations, relevant to future drug discovery and development. Exp. Opin. Invest. Drugs 9, 1553–1571 (2000).
Kaneko, H., Kaunitz, J. & Tache, Y. Vagal mechanisms underlying gastric protection induced by chemical activation of raphe pallidus in rats. Am. J. Physiol. 275, G1056–G1062 (1998).
MacLean, D. B. Abrogation of peripheral cholecystokinin-satiety in the capsaicin treated rat. Regul. Pept. 11, 321–333 (1985).
Lee, M. D., Aloyo, V. J., Fluharty, S. J. & Simansky, K. J. Infusion of the serotonin1B (5-HT1B) agonist CP-93,129 into the parabrachial nucleus potently and selectively reduces food intake in rats. Psychopharmacology 136, 304–307 (1998).
Schwartz, M. W., Woods, S. C., Porte, D. J., Seeley, R. J. & Baskin, D. G. Central nervous system control of food intake. Nature 404, 661–671 (2000).
Curran, T., Gordon, M. B., Rubino, K. L. & Sambucetti, L. C. Isolation and characterization of the c-fos (rat) cDNA and analysis of post-translational modification in vitro. Oncogene 2, 79–84 (1987).
Ritter, R. C., Covasa, M. & Matson, C. A. Cholecystokinin: proofs and prospects for involvement in control of food intake and body weight. Neuropeptides 33, 387–399 (1999).
Giuffrida, A. & Piomelli, D. in Lipid Second Messengers (eds Laychock, S. G. & Rubin, R. P.) 113–133 (CRC, Boca Raton, 1998).
Désarnaud, F., Cadas, H. & Piomelli, D. Anandamide amidohydrolase activity in rat brain microsomes. Identification and partial characterization. J. Biol. Chem. 270, 6030–6035 (1995).
Giuffrida, A., Rodríguez de Fonseca, F. & Piomelli, D. Quantification of bioactive acylethanolamides in rat plasma by electrospray mass spectrometry. Anal. Biochem. 280, 87–93 (2000).
Navarro, M. et al. Acute administration of the CB1 cannabinoid receptor antagonist SR 141716A induces anxiety-like responses in the rat. Neuroreport 8, 491–496 (1997).
Navarro, M. et al. Colocalization of glucagon-like peptide-1 (GLP-1) receptors, glucose transporter GLUT-2, and glucokinase mRNAs in rat hypothalamic cells: evidence for a role of GLP-1 receptor agonists as an inhibitory signal for food and water intake. J. Neurochem. 67, 1982–1991 (1996).
Guthrie, K. M., Anderson, A. J., Leon, M. & Gall, C. Odor-induced increases in c-fos mRNA expression reveal an anatomical “unit” for odor processing in olfactory bulb. Proc. Natl Acad. Sci. USA 90, 3329–3333 (1993).
Lauterborn, J. C., Isackson, P. J., Montalvo, R. & Gall, C. M. In situ hybridization localization of choline acetyltransferase mRNA in adult rat brain and spinal cord. Brain Res. Mol. Brain Res. 17, 59–69 (1993).
Acknowledgements
We thank M. Schwartz for comments; R. Carrera and A. M. Basso for help with initial experiments; T. Dinh, M. Guzmán and C. Sánchez for reading the manuscript critically; M. A. Gorriti, J. Muñoz, F. Désarnaud, M. A. Villanúa and Y. Xie for help with experiments; and F. Valiño for editorial assistance. This research was supported by grants from the National Institute of Drug Abuse (to D.P.) and from the National Institute of Mental Health (to C.G.). Additional support was from the Comunidad de Madrid, Del Amo Program and Plan Nacional sobre Drogas (to F.R.F. and M.N.). F.R.F. is a research fellow of the Jaime del Amo Program, Complutense University.
Author information
Authors and Affiliations
Corresponding author
Supplementary information

Supplementary Figure 1
(GIF 11.8 KB)
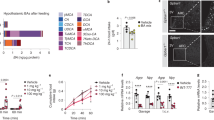
Behavioral specificity of OEA-induced hypophagia. Effects of i.p. vehicle (V) or OEA (5 or 20 mg per kg) on: a, rectal temperature; b, latency to jump in the hot plate test for analgesia; c, percent time spent in open arms in the elevated plus maze test for anxiety; d, cumulative water intake; e, number of operant responses for food. Asterisk, P < 0.05; two asterisks, P < 0.01, n = 8-12 per group.
Rights and permissions
About this article
Cite this article
Rodríguez de Fonseca, F., Navarro, M., Gómez, R. et al. An anorexic lipid mediator regulated by feeding. Nature 414, 209–212 (2001). https://doi.org/10.1038/35102582
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35102582