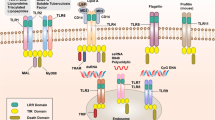
Key Points
-
Pattern-recognition receptors of the innate immune system recognize conserved pathogen-associated molecular patterns.
-
Drosophila has two main pathways of microbial recognition. The Toll pathway is involved in recognition of fungal and Gram-positive bacterial pathogens. The Imd pathway is essential for recognition and response to Gram-negative bacterial infection.
-
Mammalian Toll-like receptors (TLRs) recognize a variety of microbial products: TLR2 is specific for peptidoglycan and lipoproteins; TLR3 is specific for double-stranded RNA; TLR4 is specific for lipopolysaccharide and lipoteichoic acids; TLR5 is specific for bacterial flagellin; and TLR9 is specific for CpG DNA.
-
TLR2 cooperates with TLR1 and TLR6 for recognition of distinct sets of ligands.
-
TLRs signal through MyD88, IL-1R-associated kinase (IRAK) and TNF-receptor-associated factor 6 (TRAF6). In addition to MyD88-dependent signalling, TLR4 signals through an additional adaptor protein, TIRAP (TIR domain-containing adaptor protein).
-
TLR-mediated recognition and signalling have a crucial role in the initiation of adaptive immune responses and in the induction of T-cell differentiation into T helper (TH)1 effectors.
Abstract
Toll-like receptors have a crucial role in the detection of microbial infection in mammals and insects. In mammals, these receptors have evolved to recognize conserved products unique to microbial metabolism. This specificity allows the Toll proteins to detect the presence of infection and to induce activation of inflammatory and antimicrobial innate immune responses. Recognition of microbial products by Toll-like receptors expressed on dendritic cells triggers functional maturation of dendritic cells and leads to initiation of antigen-specific adaptive immune responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Janeway, C. A. Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 54, 1–13 (1989).This is a landmark paper that introduced the concepts of pattern recognition and the role of innate immune recognition in the control of adaptive immunity.
Janeway, C. A. Jr. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol. Today 13, 11–16 (1992).
Medzhitov, R. & Janeway, C. A. Jr. Innate immunity: impact on the adaptive immune response. Curr. Opin. Immunol. 9, 4–9 (1997).
Hashimoto, C., Hudson, K. L. & Anderson, K. V. The Toll gene of Drosophila, required for dorsal–ventral embryonic polarity, appears to encode a transmembrane protein. Cell 52, 269–279 (1988).
Medzhitov, R., Preston-Hurlburt, P. & Janeway, C. A. Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388, 394–397 (1997).
Rock, F. L., Hardiman, G., Timans, J. C., Kastelein, R. A. & Bazan, J. F. A family of human receptors structurally related to Drosophila Toll. Proc. Natl Acad. Sci. USA 95, 588–593 (1998).
Kobe, B. & Deisenhofer, J. Proteins with leucine-rich repeats. Curr. Opin. Struct. Biol. 5, 409–416 (1995).
Aravind, L., Dixit, V. M. & Koonin, E. V. Apoptotic molecular machinery: vastly increased complexity in vertebrates revealed by genome comparisons. Science 291, 1279–1284 (2001).
Muzio, M., Ni, J., Feng, P. & Dixit, V. M. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science 278, 1612–1615 (1997).
Medzhitov, R. et al. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 2, 253–258 (1998).
Wesche, H., Henzel, W. J., Shillinglaw, W., Li, S. & Cao, Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity 7, 837–847 (1997).
Burns, K. et al. MyD88, an adapter protein involved in interleukin-1 signaling. J. Biol. Chem. 273, 12203–12209 (1998).
Horng, T., Barton, G. M. & Medzhitov, R. TIRAP: an adapter molecule in the Toll signaling pathway. Nature Immunol. 2, 835–841 (2001).
Anderson, K. V. Toll signaling pathways in the innate immune response. Curr. Opin. Immunol. 12, 13–19 (2000).
Belvin, M. P. & Anderson, K. V. A conserved signaling pathway: the Drosophila Toll-dorsal pathway. Annu. Rev. Cell Dev. Biol. 12, 393–416 (1996).
Lemaitre, B., Nicolas, E., Michaut, L., Reichhart, J. M. & Hoffmann, J. A. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86, 973–983 (1996).This is a seminal study that showed the role of the Toll pathway in Drosophila immunity.
Meng, X., Khanuja, B. S. & Ip, Y. T. Toll receptor-mediated Drosophila immune response requires Dif, an NF-κB factor. Genes Dev. 13, 792–797 (1999).
Rutschmann, S. et al. The Rel protein DIF mediates the antifungal but not the antibacterial host defense in Drosophila. Immunity 12, 569–580 (2000).
Levashina, E. A. et al. Constitutive activation of Toll-mediated antifungal defense in serpin-deficient Drosophila. Science 285, 1917–1919 (1999).
Khush, R. S., Leulier, F. & Lemaitre, B. Drosophila immunity: two paths to NF-κB. Trends Immunol. 22, 260–264 (2001).
Lemaitre, B., Reichhart, J. M. & Hoffmann, J. A. Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc. Natl Acad. Sci. USA 94, 14614–14619 (1997).This study indicated that Drosophila can discriminate between different pathogen classes and induce an appropriate set of antimicrobial peptides.
Lemaitre, B. et al. A recessive mutation, immune deficiency (imd), defines two distinct control pathways in the Drosophila host defense. Proc. Natl Acad. Sci. USA 92, 9465–9469 (1995).
Imler, J. L. & Hoffmann, J. A. Signaling mechanisms in the antimicrobial host defense of Drosophila. Curr. Opin. Microbiol. 3, 16–22 (2000).
Georgel, P. et al. Drosophila immune deficiency (IMD) is a death domain protein that activates antibacterial defence and can promote apoptosis. Dev. Cell 1, 503–514 (2001)
Elrod-Erickson, M., Mishra, S. & Schneider, D. Interactions between the cellular and humoral immune responses in Drosophila. Curr. Biol. 10, 781–784 (2000).
Leulier, F., Rodriguez, A., Khush, R. S., Abrams, J. M. & Lemaitre, B. The Drosophila caspase Dredd is required to resist Gram-negative bacterial infection. EMBO Rep. 1, 353–358 (2000).
Lu, Y., Wu, L. P. & Anderson, K. V. The antibacterial arm of the Drosophila innate immune response requires an IκB kinase. Genes Dev. 15, 104–110 (2001).
Silverman, N. et al. A Drosophila IκB kinase complex required for relish cleavage and antibacterial immunity. Genes Dev. 14, 2461–2471 (2000).
Rutschmann, S. et al. Role of Drosophila IKK-γ in a Toll-independent antibacterial immune response. Nature Immunol. 1, 342–347 (2000).
Vidal, S. et al. Mutations in the Drosophila dTAK1 gene reveal a conserved function for MAPKKKs in the control of rel/NF-κB-dependent innate immune responses. Genes Dev. 15, 1900–1912 (2001).
Hedengren, M. et al. Relish, a central factor in the control of humoral but not cellular immunity in Drosophila. Mol. Cell 4, 827–837 (1999).
Chen, P., Rodriguez, A., Erskine, R., Thach, T. & Abrams, J. M. Dredd, a novel effector of the apoptosis activators reaper, grim, and hid in Drosophila. Dev. Biol. 201, 202–216 (1998).
Karin, M. & Delhase, M. The IκB kinase (IKK) and NF-κB: key elements of proinflammatory signalling. Semin. Immunol. 12, 85–98 (2000).
Dushay, M. S., Asling, B. & Hultmark, D. Origins of immunity: Relish, a compound Rel-like gene in the antibacterial defense of Drosophila. Proc. Natl Acad. Sci. USA 93, 10343–10347 (1996).
Tauszig, S., Jouanguy, E., Hoffmann, J. A. & Imler, J. L. From the cover: Toll-related receptors and the control of antimicrobial peptide expression in Drosophila. Proc. Natl Acad. Sci. USA 97, 10520–10525 (2000).
Williams, M. J., Rodriguez, A., Kimbrell, D. A. & Eldon, E. D. The 18-wheeler mutation reveals complex antibacterial gene regulation in Drosophila host defense. EMBO J. 16, 6120–6130 (1997).
Akira, S., Takeda, K. & Kaisho, T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nature Immunol. 2, 675–680 (2001).
Poltorak, A. et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 (1998).
Qureshi, S. T. et al. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J. Exp. Med. 189, 615–625 (1999); erratum 189, 1518 (1999). | PubMed |
Hoshino, K. et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162, 3749–3752 (1999).References 38–40 describe the first indication of TLR4 function in vivo.
Wright, S. D., Tobias, P. S., Ulevitch, R. J. & Ramos, R. A. Lipopolysaccharide (LPS) binding protein opsonizes LPS-bearing particles for recognition by a novel receptor on macrophages. J. Exp. Med. 170, 1231–1241 (1989).
Wright, S. D., Ramos, R. A., Tobias, P. S., Ulevitch, R. J. & Mathison, J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249, 1431–1433 (1990).
Haziot, A. et al. Resistance to endotoxin shock and reduced dissemination of Gram-negative bacteria in CD14-deficient mice. Immunity 4, 407–414 (1996).
Shimazu, R. et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189, 1777–1782 (1999).
Schromm, A. B. et al. Molecular genetic analysis of an endotoxin nonresponder mutant cell line. A point mutation in a conserved region of md-2 abolishes endotoxin-induced signaling. J. Exp. Med. 194, 79–88 (2001).
Lien, E. et al. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J. Clin. Invest. 105, 497–504 (2000).
Poltorak, A., Ricciardi-Castagnoli, P., Citterio, S. & Beutler, B. Physical contact between lipopolysaccharide and Toll-like receptor 4 revealed by genetic complementation. Proc. Natl Acad. Sci. USA 97, 2163–2167 (2000).
Da Silva Correia, J., Soldau, K., Christen, U., Tobias, P. S. & Ulevitch, R. J. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex. Transfer from CD14 to TLR4 and MD-2. J. Biol. Chem. 276, 21129–21135 (2001).
Miyake, K., Yamashita, Y., Ogata, M., Sudo, T. & Kimoto, M. RP105, a novel B cell surface molecule implicated in B cell activation, is a member of the leucine-rich repeat protein family. J. Immunol. 154, 3333–3340 (1995).
Chan, V. W. et al. The molecular mechanism of B cell activation by Toll-like receptor protein RP-105. J. Exp. Med. 188, 93–101 (1998).
Miyake, K. et al. Mouse MD-1, a molecule that is physically associated with RP105 and positively regulates its expression. J. Immunol. 161, 1348–1353 (1998).
Ogata, H. et al. The Toll-like receptor protein RP105 regulates lipopolysaccharide signaling in B cells. J. Exp. Med. 192, 23–29 (2000).
Takeuchi, O. et al. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cel wall components. Immunity 11, 443–451 (1999).
Means, T. K. et al. Human Toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J. Immunol. 163, 3920–3927 (1999).
Ohashi, K., Burkart, V., Flohe, S. & Kolb, H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the Toll-like receptor-4 complex. J. Immunol. 164, 558–561 (2000).
Li, M. et al. An essential role of the NF-κB/Toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J. Immunol. 166, 7128–7135 (2001).
Kurt-Jones, E. A. et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nature Immunol. 1, 398–401 (2000).
Bowie, A. et al. A46R and A52R from vaccinia virus are antagonists of host IL-1 and Toll-like receptor signaling. Proc. Natl Acad. Sci. USA 97, 10162–10167 (2000).
Schwandner, R., Dziarski, R., Wesche, H., Rothe, M. & Kirschning, C. J. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J. Biol. Chem. 274, 17406–17409 (1999).
Takeuchi, O. et al. Cutting edge: preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a Toll-like receptor 2- and MyD88-dependent signaling pathway. J. Immunol. 164, 554–557 (2000).
Aliprantis, A. O. et al. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science 285, 736–739 (1999).
Brightbill, H. D. et al. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science 285, 732–736 (1999).
Means, T. K. et al. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J. Immunol. 163, 6748–6755 (1999).
Underhill, D. M., Ozinsky, A., Smith, K. D. & Aderem, A. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc. Natl Acad. Sci. USA 96, 14459–14463 (1999).
Campos, M. A. et al. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J. Immunol. 167, 416–423 (2001).
Hajjar, A. M. et al. Cutting edge: functional interactions between Toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J. Immunol. 166, 15–19 (2001).
Underhill, D. et al. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401, 811–815 (1999).
Werts, C. et al. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nature Immunol. 2, 346–352 (2001).
Hirschfeld, M. et al. Signaling by Toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect. Immun. 69, 1477–1482 (2001).
Ozinsky, A. et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc. Natl Acad. Sci. USA 97, 13766–13771 (2000).
Takeuchi, O. et al. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 13, 933–940 (2001).
Muzio, M. et al. Differential expression and regulation of Toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J. Immunol. 164, 5998–6004 (2000).
Alexopoulou, L., Holt, A. C., Medzhitov, R. & Flavell, R. A. Recognition of double stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413, 732–738 (2001).
Williams, B. R. PKR; a sentinel kinase for cellular stress. Oncogene 18, 6112–6120 (1999).
Chu, W. M. et al. JNK2 and IKKβ are required for activating the innate response to viral infection. Immunity 11, 721–731 (1999).
Hayashi, F. et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410, 1099–1103 (2001).
Samatey, F. A. et al. Structure of the bacterial flagellar protofilament and implications for a switch for supercoiling. Nature 410, 331–337 (2001).
Gewirtz, A. T., Navas, T. A., Lyons, S., Godowski, P. J. & Madara, J. L. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167, 1882–1885 (2001).
Hemmi, H. et al. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000).
Krieg, A. M. et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374, 546–549 (1995).
Hacker, H. et al. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J. 17, 6230–6240 (1998).
Thieblemont, N. & Wright, S. D. Transport of bacterial lipopolysaccharide to the Golgi apparatus. J. Exp. Med. 190, 523–534 (1999).
Krieg, A. M. The role of CpG motifs in innate immunity. Curr. Opin. Immunol. 12, 35–43 (2000).
Bauer, S. et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl Acad. Sci. USA 98, 9237–9242 (2001).
Thoma-Uszynski, S. et al. Induction of direct antimicrobial activity through mammalian Toll-like receptors. Science 291, 1544–1547 (2001).
Burns, K. et al. Tollip, a new component of the IL-1RI pathway, links IRAK to the IL-1 receptor. Nature Cell Biol. 2, 346–351 (2000).
Bulut, Y., Faure, E., Thomas, L., Equils, O. & Arditi, M. Cooperation of Toll-Like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and Borrelia burgdorferi outer surface protein A lipoprotein: role of Toll-interacting protein and IL-1 receptor signaling molecules in Toll-like receptor 2 signaling. J. Immunol. 167, 987–994 (2001).
Cao, Z., Henzel, W. J. & Gao, X. IRAK: a kinase associated with the interleukin-1 receptor. Science 271, 1128–1131 (1996).
Cao, Z., Xiong, J., Takeuchi, M., Kurama, T. & Goeddel, D. V. TRAF6 is a signal transducer for interleukin-1. Nature 383, 443–446 (1996).
Lomaga, M. A. et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 13, 1015–1024 (1999).
Adachi, O. et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9, 143–150 (1998).
Kawai, T., Adachi, O., Ogawa, T., Takeda, K. & Akira, S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11, 115–122 (1999).
Wang, C. et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412, 346–351 (2001).
Arbibe, L. et al. Toll-like receptor 2-mediated NF-κB activation requires a Rac1-dependent pathway. Nature Immunol. 1, 533–540 (2000).
Schnare, M., Holt, A. C., Takeda, K., Akira, S. & Medzhitov, R. Recognition of CpG DNA is mediated by signaling pathways dependent on the adaptor protein MyD88. Curr. Biol. 10, 1139–1142 (2000).
Kaisho, T., Takeuchi, O., Kawai, T., Hoshino, K. & Akira, S. Endotoxin-induced maturation of Myd88-deficient dendritic cells. J. Immunol. 166, 5688–5694 (2001).
Seki, E. et al. Lipopolysaccharide-induced IL-18 secretion from murine Kupffer cells independently of myeloid differentiation factor 88 that is critically involved in induction of production of IL-12 and IL-1β. J. Immunol. 166, 2651–2657 (2001).
Fitzgerald, K. A. et al. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature 413, 78–83 (2001).
Goh, K. C., deVeer, M. J. & Williams, B. R. The protein kinase PKR is required for p38 MAPK activation and the innate immune response to bacterial endotoxin. EMBO J. 19, 4292–4297 (2000).
Banchereau, J. & Steinman, R. M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Schnare, M. et al. Toll-like receptors control activation of adaptive immune responses. Nature Immunol. 2, 947–950 (2001).
Holmskov, U. L. Collectins and collectin receptors in innate immunity. APMIS Suppl. 100, 1–59 (2000).
Gewurz, H., Mold, C., Siegel, J. & Fiedel, B. C-reactive protein and the acute phase response. Adv. Intern. Med. 27, 345–372 (1982).
Schwalbe, R. A., Dahlback, B., Coe, J. E. & Nelsestuen, G. L. Pentraxin family of proteins interact specifically with phosphorylcholine and/or phosphorylethanolamine. Biochemistry 31, 4907–4915 (1992).
Fraser, I. P., Koziel, H. & Ezekowitz, R. A. The serum mannose-binding protein and the macrophage mannose receptor are pattern recognition molecules that link innate and adaptive immunity. Semin. Immunol. 10, 363–372 (1998).
Pearson, A. M. Scavenger receptors in innate immunity. Curr. Opin. Immunol. 8, 20–28 (1996).
Elomaa, O. et al. Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell 80, 603–609. (1995).
Inohara, N. et al. Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-κB. J. Biol. Chem. 274, 14560–14567 (1999).
Bertin, J. et al. Human CARD4 protein is a novel CED-4/Apaf-1 cell death family member that activates NF-κB. J. Biol. Chem. 274, 12955–12958 (1999).
Author information
Authors and Affiliations
Related links
Related links
DATABASES
Glossary
- TYPE III SECRETION SYSTEM
-
A specialized multisubunit secretion apparatus found in many Gram-negative bacterial pathogens. It allows the bacteria to secrete various effector proteins directly into the cytosol of the host cells, where they have several functions, such as induction of apoptosis and stimulation of phagocytosis.
- DEATH DOMAIN
-
A protein–protein interaction domain found in many proteins that are involved in signalling and apoptosis.
- ENDOTOXIC SHOCK
-
A clinical condition induced by hyper-reaction of the innate immune system to bacterial LPS. It is mediated by the inflammatory cytokines IL-1 and TNF-α, which are produced in high amounts due to sustained stimulation of TLR4 by LPS.
- COMPLETE FREUND'S ADJUVANT
-
(CFA). A mixture of mycobacterial lysate with mineral oil. When animals are immunized with antigen mixed with CFA, they induce strong immune responses to the antigen.
Rights and permissions
About this article
Cite this article
Medzhitov, R. Toll-like receptors and innate immunity. Nat Rev Immunol 1, 135–145 (2001). https://doi.org/10.1038/35100529
Issue Date:
DOI: https://doi.org/10.1038/35100529
This article is cited by
-
Emerging strategies for treating autoimmune disease with genetically modified dendritic cells
Cell Communication and Signaling (2024)
-
Pulmonary toxicity assessment of polypropylene, polystyrene, and polyethylene microplastic fragments in mice
Toxicological Research (2024)
-
Cellular and molecular mechanisms of cell damage and cell death in ischemia–reperfusion injury in organ transplantation
Molecular Biology Reports (2024)
-
The role of CD180 in hematological malignancies and inflammatory disorders
Molecular Medicine (2023)
-
The architecture of transmembrane and cytoplasmic juxtamembrane regions of Toll-like receptors
Nature Communications (2023)