Key Points
-
Most antigens enter the body at mucosal surfaces. Unique adaptations support the barrier function of the intestinal epithelium.
-
The gut-associated lymphoid tissue (GALT) is divided into discrete inductive (Peyer's patch and mesenteric lymph node; MLN) and effector (lamina propria) sites that contain specialized populations of B (IgA) and T cells.
-
The accumulation of memory T-effector cells at epithelial surfaces 'man the barriers' for strategic mucosal defense.
-
Toll-like receptors (TLRs) recognize microbial structures that are common to both pathogens and commensals. Although commensals can gain access to the GALT, only pathogens elicit an adaptive immune response.
-
The intestinal epithelium has an important role in orchestrating immune responses in the GALT by generating both pro-inflammatory and anti-inflammatory signals.
-
The factors controlling responsiveness to pathogens and nonresponsiveness to innocuous antigens from food or commensals are delicately balanced. Mucosal dysregulation leads to chronic intestinal inflammation.
-
Food proteins typically induce systemic nonresponsiveness (oral tolerance). As in the periphery, dendritic cells (DCs) have a primary role in the presentation of antigen to naive T cells in the GALT.
-
Nonresponsiveness to food proteins is originally manifested as a reduction in the proliferative capacity of antigen-specific T cells in the draining MLN. This is likely to be due to the preferential binding of cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), to low levels of the co-stimulatory molecules CD80/CD86 in the absence of inflammatory signals.
-
High-level production of anti-inflammatory mediators, such as interleukin-10 and prostaglandin E2, in the GALT make it a headquarters for the generation of regulatory T cells.
Abstract
Immunologists typically study the immune responses induced in the spleen or peripheral lymph nodes after parenteral immunization with antigen and poorly defined experimental adjuvants. However, most antigens enter the body through mucosal surfaces. It is now clear that the microenvironment in these mucosal barriers has a marked influence on the immune response that ultimately ensues. Nowhere is the microenvironment more influential than in the gut-associated lymphoid tissue (GALT). The GALT must constantly distinguish harmless antigens that are present in food or on commensal bacteria from pathogenic assault by microbes. It is perhaps not surprising, then, that the GALT contains more lymphocytes than all of the secondary lymphoid organs combined.
Similar content being viewed by others
Main
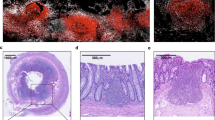
Mucosal epithelia are primary sites for antigen entry. Afferent lymphatics carry antigens from the mucosal surface to draining lymph nodes where specialized antigen-presenting cells (APCs) present them to the immune system. Of the mucosal lymphoid tissues, the organized lymphoid tissues of the respiratory and gastrointestinal tracts contain the largest numbers of lymphocytes, and are the most fully characterized (Fig. 1). Other mucosal surfaces, such as those of the urogenital tract, also contain populations of mucosal lymphocytes that are important in immune defence and the protection of the epithelial barrier.
Inhaled or ingested antigens enter the body through the mucosal surfaces of the respiratory and gastrointestinal tracts. Both diffusely scattered lymphocytes and organized lymphoid follicles (for example, the Peyer's patches) line the mucosal surfaces. Antigens are transported from the mucosa to draining lymph nodes (mediastinal or mesenteric) by afferent lymphatics and presented to T cells in the node. Antigen-sensitized T cells can then migrate out of the draining node through efferent lymphatics and ultimately enter the systemic circulation through the thoracic duct.
Although consisting of only a single layer of cells, the intestinal epithelium must control the access of potential antigens and pathogens, and, at the same time, function in the digestive absorption of dietary nutrients. It is aided this dual role by intercellular TIGHT JUNCTIONS that restrict the passage of even very small (2-kDa) molecules1. The intestinal epithelium also boasts a number of specialized protective adaptations that are not found in other sites. These include: anti-microbial peptides (DEFENSINS)2, secretory immunoglobulin A3,4, mucins and TREFOIL PEPTIDES5. The apical surface of the enterocyte, which faces the intestinal lumen, is ideally suited for the terminal digestion of nutrients because of its dense coating with absorptive microvilli. At the tips of the microvilli, a layer of membrane-anchored glycoproteins forms the filamentous brush border glycocalyx (FBBG)6. Digested nutrients can gain access to the body through the FBBG, but it is relatively impermeant to macromolecules or bacteria.
How do antigens get in?
Soluble proteins and microbes do cross the epithelial barrier. Enterocytes are the primary cell type in the epithelial monolayer, which is interspersed in some regions by a specialized epithelial cell type known as the M cell. The brush border glycocalyx that characterizes villus enterocytes is absent from the apical surface of the M cell. It is replaced by microfolds (hence 'M cell') that are more accessible to luminal antigens. M cells use transepithelial vesicular transport to carry microbes to APCs in the underlying gut-associated lymhoid tissue (GALT)6. The GALT is divided into discrete inductive and effector sites (Fig. 2). M cells are contained within inductive sites in the GALT, known as the Peyer's patches. Peyer's patches are aggregations of lymphoid follicles found primarily in the distal ileum of the small intestine. Located within the dome-like structure of the follicle-associated epithelium, M cells have long been thought to act as 'gateways to the mucosal immune system', delivering antigen to APCs in the Peyer's patch subepithelial dome7. It turns out, however, that M cells are not the only cell type capable of transporting antigen across the epithelial barrier. A recent report indicates that one type of professional APC, the dendritic cell (DC), might extend its dendrite-like processes through epithelial tight junctions and sample luminal antigen directly8. The integrity of the barrier would be maintained during this process because tight junctions are re-formed by proteins that are expressed on both enterocytes and the DCs themselves.
The gut-associated lymphoid tissue (GALT) is divided into inductive (Peyer's patch) and effector (lamina propria) sites. In the immunofluorescence images shown, T cells are green and dendritic cells (DCs) are red. a | Similar to lymph nodes, the Peyer's patch contains B-cell follicles. The follicle-associated epithelium (FAE) covers the dome of the Peyer's patch. Transport across the epithelium occurs through both specialized M cells and by DCs that extend their processes through epithelial tight junctions. DCs are present in both the subepithelial dome (SED) and the interfollicular T-cell areas and are visible as stellate (red) cells in these sites. b | The intestinal villus epithelium contains an unusual population of intraepithelial lymphocytes (IELs) which reside above the epithelial basement membrane. Scattered lamina propria (LP) effector cells — T cells (T), IgA-secreting B cells (B) and DCs — are located within the villi. (Immunofluorescent images courtesy of E. I. Melendro, Massachusetts General Hospital.)
Specialized immunoglobulin and T cells
The aggregations of lymphoid follicles that form the Peyer's patches are the most readily discernible of the follicles dispersed throughout the small and large intestine. Like lymph nodes, Peyer's patches have B-cell follicles and germinal centres that are surrounded by areas that contain predominantly T cells. The GALT also contains loosely organized effector sites, primarily within the lamina propria of the intestinal villi (Fig. 2). The lymphocytes found in the lamina propria are largely IgA-secreting plasma cells and memory T-effector cells9. IgA is a 'non-inflammatory' form of immunoglobulin in that it binds complement weakly, if at all10. Although produced in enormous quantity at the mucosal surface, much less IgA is found in the circulation. Moreover, systemic IgA circulates as a monomer, whereas mucosal IgA is typically secreted as a dimer. Binding to the polymeric immunoglobulin receptor (pIgR) that is expressed constitutively on the basolateral surface of enterocytes results in the transport of IgA to the apical surface. At the surface, the portion of the pIgR attached to the Fc (crystallizable fragment) region of IgA is enzymatically cleaved and remains bound to the dimeric IgA molecule as the SECRETORY COMPONENT, which is thought to help prevent proteolytic damage to secretory IgA in the harsh luminal environment (reviewed in Ref. 11). The role of secretory IgA in excluding antigen from entering the epithelium has long been appreciated. It has recently been suggested that IgA also runs a shuttle service, using the pIgR to actively transport antigen out of the lamina propria to the apical surface of the enterocyte4. These observations indicate a twofold barrier function for secretory IgA, both in guarding the epithelium from microbial entry and as a lamina propria 'sump pump', excreting IgA-bound antigens or microbes that penetrate the barrier back into the lumen.
Immunoglobulin production typically requires T-cell help. CD4+ helper T (TH) cells are divided into two functional subsets on the basis of their pattern of cytokine secretion. In general, TH1 cells produce interferon-γ (IFN-γ), which is important for cell-mediated immune responses and inflammation. TH2 cells secrete interleukin (IL)-4, IL-5 and IL-13, and induce B-cell activation and differentiation. Unlike other immunoglobulin isotypes, signals through TRANSFORMING GROWTH FACTOR-B (TGF-β)/TGF-β receptor type II on B cells are vital for class switching to IgA12. TH2 cytokines then control B-cell differentiation into IgA-secreting plasma cells13. TGF-β is abundantly expressed in the GALT and is central to two of the most distinctive functions of GALT: secretion of IgA and the generation of regulatory T cells14 (see below). Interestingly, non-bone-marrow-derived stromal cells in the lamina propria also contribute to the anti-inflammatory tone of the GALT through their secretion of prostaglandin E2 (PGE2)15,16. The spontaneous and constitutive production of the enzyme cyclooxygenase 2 (COX2), which metabolizes PGE2, is not dependent on the presence of the luminal flora or on inflammatory stimuli. Instead, high-level production of ARACHIDONIC ACID METABOLITES is a stable basal phenotype that is unique to the intestinal lamina propria and might represent one of the earliest developmental adaptations in establishing the anti-inflammatory environment of the GALT.
In addition to employing its own representative of the humoral immune response, the GALT contains some unusual T cells. Indeed, the intestinal epithelium might be unique in its ability to function as an important site for the extrathymic maturation of a substantial population of T cells. Recent work in the mouse has shown that, throughout the small and large intestine, clusters of T-cell progenitors are found in the crypt lamina propria17. These CRYPTOPATCHES are indispensable for the thymus-independent development of intra-epithelial lymphocytes (IELs), a subpopulation of T cells which resides between epithelial cells, above the basement membrane. The enterocytes themselves produce IL-7, which is important for the development of these IELs18. Expression of CD8αα homodimers, rather than the CD8αβ heterodimer that is expressed by CD8+ T cells in other sites, identifies extrathymically derived IELs. However, no evidence has been obtained for a functional role for the CD8αα chain in these IELs, which include most γδTCR+ IELs and many αβTCR+ IELs that develop without passing through the thymus. Infection by a large variety of pathogens results in the activation of IELs, but, so far, no defensive role has been ascribed to the extrathymically derived CD8αα+ subset.
Memory T cells accumulate in the GALT
Recent work has shown that CD8+ memory T-effector cells, generated as the result of bacterial or viral infection, accumulate in non-lymphoid tissues, particularly in the lamina propria, awaiting the next antigenic challenge19,20. Similarly, intravenous injection of soluble antigen (with bacterial lipopolysaccharide (LPS) as an inflammation-inducing adjuvant) also leads to the accumulation of CD4+ effector cells in the lamina propria21. The memory cells that accumulate at non-lymphoid sites are thought to belong to the 'effector memory' subset (described by Sallusto and colleagues22), and are distinguished from the 'central-memory' cells found in lymphoid organs by the latter's expression of the chemokine (C-C motif) receptor CCR7. Recent work has identified chemokine–receptor pairs which regulate tissue-specific migration. One such pair is thymocyte-expressed chemokine (TECK, CCL25) and its ligand CCR9. CCL25 is selectively expressed in the thymus (where it attracts developing CCR9+ thymocytes to the thymic epithelium) and in the small (but not large) intestine in both mice23,24 and humans25,26. CCR9 is present on virtually all CD4+ and CD8+ lymphocytes in the small intestine24,25. In the mouse, preferential expression of CCL25 in the villous crypts also indicates a role in the extrathymic maturation of IELs23. The dual expression of the intestinal homing receptor α4β7 (an integrin which binds the mucosal VASCULAR ADDRESSIN, mucosal addressin cell-adhesion molecule 1) and CCR9 allows for the selective homing of memory T cells to the lamina propria of the small intestine. Memory T-effector cells respond more rapidly to antigen challenge than the central memory cells that are localized in lymphoid organs. The ability of GALT to respond quickly and effectively to repeated assault by enteric pathogens is clearly enhanced by the accumulation of memory T-effector cells at this site. Antigen challenge seems to redistribute memory T-effector cells to 'man the barrier' for strategic mucosal defence.
The Toll of setting the tone
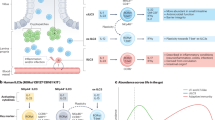
Given that an estimated 400 different commensal microbial species populate the intestinal lumen27, how does the GALT distinguish pathogens from the normal luminal flora? Virulence genes in pathogenic microbes facilitate their colonization, invasion and subsequent intracellular survival. Many pathogens are transported into the Peyer's patch through M cells. Once the epithelial barrier is breached, a rapid host response is required to confine the invaders to the mucosa. This job falls to the innate immune system. Microbes initiate a host-protective response by activating an evolutionarily conserved, primitive pattern-recognition system28. One class of pattern-recognition receptors for these pathogen-associated molecular patterns (PAMPs) was originally identified as Toll receptors in Drosophila. Some of their mammalian counterparts, the Toll-like receptors (TLRs), have recently been described (reviewed in Ref. 29). Macrophages and DCs waiting just below the epithelial dome are well positioned to detect microbial entry into the Peyer's patch30 (Box 1). Signalling by TLRs would then induce the upregulation of the co-stimulatory molecules that provide the requisite 'second signal' for the activation of naive T cells31. However, the essential microbial structural elements that are recognized by these TLRs are common to both the commensal flora and pathogens. Why don't commensal bacteria in the luminal flora continuously activate the innate immune system by signalling through TLRs, leading to constant inflammation? Part of the explanation might be that, for the most part, commensals do not gain access to the Peyer's patch. They remain trapped in the mucus layer overlying the epithelium and are blocked from reaching the surface by the physical barrier provided by the FBBG. Indeed, by their numbers alone, the normal flora are crucial in blocking pathogens from gaining access to the epithelial surface32. The answer cannot lie, however, simply in the physical exclusion of commensals by the epithelium itself. In the mouse, a mechanism for the induction of a mucosal IgA response to commensal bacteria that requires neither T-cell help nor organized follicular lymphoid tissue has been described3. Plasma cells that secrete IgA specific for antigens in the cell walls of commensal bacteria are diffusely distributed throughout the intestinal lamina propria. The anti-commensal IgA response requires the presence of the intestinal flora and seems to represent an evolutionarily ancient form of specific mucosal defence. It is now apparent that this T-cell-independent IgA pathway is present even in μMT mice, which lack the transmembrane form of IgM33. μMT mice have a block in B-cell development at the pro-B stage, as a transmembrane immunoglobulin heavy chain is required for the survival, proliferation and further differentiation of pre-B cells. The work of Zinkernagel and co-workers33 indicates the existence of an alternative B-cell-differentiation pathway that is characterized by the very early expression of the immunoglobulin α-heavy chain. The presence of these cells predominantly in the GALT indicates that the switch to IgA (and not other immunoglobulin isotypes) requires a signal derived from the gut, possibly TGF-β12. T-cell-independent IgA-secreting B cells then seem to be maintained and driven by the continued presence of commensal antigens.
How do commensal antigens gain access to the GALT? The recent observation that DCs can extend their processes between epithelial tight junctions and sample luminal microbes indicate a readily accessible route for commensal entry8,34. Rescigno and colleagues have also shown that both pathogenic and non-pathogenic bacteria are taken up by DCs that are recruited into the epithelium. However, DCs carrying non-invasive bacteria are not observed deeper in the intestinal villi, indicating that they remain in the lamina propria8. This implies that pathogenic bacteria might induce the maturation and migration of DCs, whereas commensals do not. The ability of DCs to discriminate between pathogens and commensals might be related, in part, to differential signalling by TLRs or groups of TLRs. The cytokine signalling abilities of the known TLRs are not equivalent. Some combinatorial TLR pairings elicit distinct cytokine programmes, indicating a mechanism by which the cytokines induced might be tailored to the class of microbe being recognized35. It could be hypothesized that commensals bear an as yet unidentified PAMP that elicits an anti-inflammatory cytokine programme or, conversely, lacks a PAMP that is related to invasiveness and that induces inflammatory cytokine production.
In the intestinal mucosa, professional APCs in the Peyer's Patches and lamina propria are not the only cells that express TLRs. Intestinal epithelial cells also possess both TLRs and the capacity to secrete a wide variety of cytokines and chemokines36,37,38,39. Whether signalling by TLRs on enterocytes leads directly to their cytokine secretion is not yet clear. In some model epithelia, bacterial LPS can activate the nuclear transcription factor NF-κB, the master switch for the cytokine-secretion programme that characterizes an inflammatory response40. If this is also true in the intestinal mucosa, inflammatory cytokines might turn on macrophage microbicidal activity, inducing the production of reactive oxygen and reactive nitrogen intermediates. The subsequent adaptive immune response is also orchestrated by epithelial-cell-derived chemokines. In the small intestine, enterocytes, particularly those in the follicle-associated epithelium, secrete the chemokine macrophage inflammatory protein 3-α (MIP-3α; CCL20)41,42,43. CCL20 specifically recruits immature, CCR6+, CD11b+ myeloid DCs to the subepithelial dome of the Peyer's patch where they function in the uptake of microbial antigen. Mutant mice lacking CCR6 have an impaired humoral immune response to enteric viral infection and to orally administered antigen plus adjuvant41.
In addition to its pro-inflammatory role, emerging evidence indicates that the intestinal epithelium is directly involved in setting the anti-inflammatory tone of the GALT. Despite the presence of large numbers of bacteria in the intestinal lumen, neutrophils are seldom found in the healthy intestinal mucosa. Although, as mentioned earlier, the physical barriers at the epithelial surface exclude most commensals, some are able to make it through. In an in vitro model, binding of non-pathogenic bacteria to the epithelium suppressed the transcription of pro-inflammatory cytokines by blocking the NF-κB/IκB PATHWAY44. Bacterial binding apparently blocks inhibitory κB (IκB) degradation, preventing the release of NF-κB and its translocation into the nucleus. These observations indicate that signalling by commensals might keep this master switch turned off until pathogen recognition turns it on.
Mucosal inflammation
The risk of widespread inflammation of the intestinal mucosa is constant. Animal models have shown just how important precise regulation can be in this delicately balanced microenvironment. Mutations in a number of immunoregulatory mediators can induce a chronic inflammatory response that is largely confined to the gut and attributable to the induction of a response to commensal flora that were previously tolerated. Chronic intestinal inflammation has been documented as a spontaneous occurrence in a number of mouse strains, as well as in a wide range of mice that are mutant for immunoregulatory cytokines45,46. Although phenotypically variable, what these models have in common is that disease is ameliorated when the mice are transferred to a germ-free environment, which eliminates the indigenous luminal flora46. In mouse models of inflammatory bowel disease (IBD), the commensals apparently turn on (and amplify) the pro-inflammatory response that they had previously suppressed. These animal models are also beginning to show how disease occurs in patients with IBD, particularly CROHN'S DISEASE. In genetically susceptible individuals, the chronic intestinal inflammation characteristic of Crohn's disease is the result of a dysregulated, chronic T-cell inflammatory response to bacteria that are normally present in the intestinal lumen47. Mutations in a gene on chromosome 16, NOD2 , have recently been associated with a subset of Crohn's disease patients48,49,50. NOD2 is a putative apoptosis regulator, and is expressed exclusively in monocytes. In individuals with this mutation, NOD2 seems to alter NF-κB signalling. However, the functional consequences of this mutation, and the means by which it initiates chronic inflammation, are controversial and not yet understood51.
Tolerance to food
Apart from commensal bacteria, the other main source of potential antigenic stimulation for the GALT comes from food proteins. A large body of experimental evidence has shown that oral administration of soluble antigens induces systemic non-responsiveness to peripheral antigen challenge52. Typically known as oral tolerance, the induction of non-responsiveness to dietary antigens is likely to have a vital physiological role in preventing hypersensitivity reactions to food (reviewed in Ref. 53). Often described as systemic non-responsiveness accompanied by local, mucosal (IgA) immunity, new evidence indicates that the predominant mucosal response to non-pathogenic luminal antigen (dietary or commensal) is also one of tolerance54,55. Although the phenomenon of oral tolerance has been known for almost a century52, many unanswered questions remain concerning its mechanistic basis. For example, is oral tolerance due solely to systemic distribution of antigen through the bloodstream, or is there a unique role for antigen presentation in the GALT? Where (and by which cells) is antigen first presented to the immune system? Does oral tolerance differ from other forms of peripheral tolerance?
Tolerance versus immunity
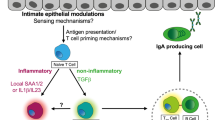
Antigen-specific responses require that the APCs provide at least two signals: antigenic peptide presented in the context of class I or class II major histocompatibility complex (MHC) and a co-stimulatory signal generated by the innate immune system. Members of the best-characterized co-stimulatory family, B7-1 and B7-2 (CD80 and CD86, respectively), bind the T-cell counter-receptors CD28 and cytotoxic T-lymphocyte antigen 4 (CTLA-4; CD152). Binding of CD80/CD86 to CD28 or CTLA-4 leads to different functional consequences: signalling through CD28 elicits a positive signal for T-cell activation and proliferation, whereas signalling through CTLA-4 results in an inhibitory signal. The high-affinity T-cell ligand CTLA-4 preferentially binds to APCs that express low levels of CD80/CD86 (Ref. 56). Consequently, non-inflammatory soluble proteins, which do not trigger the innate immune system, are presented in the presence of low levels of co-stimulatory signals and induce non-responsiveness57. By contrast, inflammatory stimuli upregulate CD80/CD86 expression, and induce the maturation and migration of DCs to the T-cell areas of lymph nodes where antigen presentation to CD28+ T cells initiates the clonal expansion that is required for the induction of an adaptive immune response.
Earlier studies emphasized a role for enterocytes in the presentation of soluble antigen and the induction of oral tolerance58. More recent evidence indicates that, in the GALT, as in other peripheral sites, DCs present antigen to naive T cells in the draining (mesenteric) lymph node and therefore govern the induction of tolerance and immunity59. As noted above, orally administered soluble proteins typically induce antigen-specific non-responsiveness. However, when this normally tolerogenic form of antigen is given in the context of a mucosal adjuvant (Box 2) or administered in a bacterial vaccine construct, the innate immune system is activated and an immune response ensues60. Interestingly, several studies have recently shown that, in the GALT, the initial response to tolerogenic or immunogenic forms of orally administered antigen is the same: transient T-cell activation and proliferation61,62,63. We have shown that, on subsequent peripheral exposure to antigen, the proliferative capacity of antigen-specific cells from mice that were fed tolerogenic antigen is reduced compared with mice that receive oral antigen administered with a mucosal adjuvant63. In the orally tolerized mice, antigen-specific clones then fail to expand and they either become anergic or die, as originally suggested by others64,65. In a similar in vivo model of tolerance, Greenwald et al.66 have recently noted that non-responsiveness is linked to the reduction in proliferative capacity induced by the peripheral administration of soluble antigen. They have shown that CTLA-4−/− TCR transgenic T cells are resistant to peripheral tolerance induction in vivo as a result of their inability to downregulate cell-cycle progression through CTLA-4 signalling. Collectively, these studies support the idea that non-responsiveness to orally administered soluble antigen is due, in part, to antigen presentation at low levels of co-stimulation and the preferential binding of CD80/CD86 by CTLA-4. Experiments are in progress to directly link CTLA-4 signalling to non-responsiveness to orally administered antigen.
As mentioned earlier, the migration of DCs has typically been associated with inflammatory stimuli that induce their maturation from an immature form, optimized for antigen uptake, to a mature form that functions primarily to present antigen to T cells in the draining lymph node. New evidence indicates, however, that in the gut, immature mucosal DCs continually pick up remnants of apoptotic enterocytes (shed from the villus tips as the epithelium regenerates) and transport them to the T-cell areas of the mesenteric lymph node67. DCs also constitutively transport antigen from the mucosal epithelium of the lungs in the absence of overt inflammatory stimulation68,69. Differential chemokine receptor expression might determine whether DCs home to inflammatory sites or to the draining lymph node69. How might DCs pick up soluble antigens without inducing the upregulation of CD80/CD86 (and DC maturation)? One possibility involves luminal antigen sampling by DCs that extend their dendrites between enterocytes8. If these DCs also constitutively migrate from the mucosal epithelium to the mesenteric lymph node (or other peripheral sites), 'immature,' gut-derived DCs might be able to induce tolerance by presenting antigen themselves or by transferring antigen to a 'tolerance-inducing' subset of DCs that are resident in T-cell areas of the lymph node70. Mature DCs might also have a role in the induction of mucosal tolerance. A recent study has shown that, in the lung, phenotypically mature (CD80/CD86hi) DCs transiently produce IL-10 and induce the development of IL-10-producing regulatory T cells71. IL-10 production by DCs was vital for the induction of tolerance to respiratory exposure to antigen in this model. M cells are not likely to be the principal pathway for transepithelial transport of soluble antigen as some recent studies in mice that are genetically deficient in Peyer's patches and M cells have shown that neither are required for the induction of oral tolerance72,73.
DCs can be broadly divided into two subsets that differ phenotypically and functionally74. Myeloid (CD11c+CD11b+) DCs are localized predominantly in the sub-epithelial dome of the Peyer's patch (Fig. 2)75, but are also found in the marginal zone of the spleen and the subcapsular sinus of lymph nodes. Lymphoid (CD11c+CD8α+) DCs are found in the T-cell areas of lymphoid organs. In the mouse, lymphoid DCs are associated with the induction of a TH1-type (inflammatory) response, whereas myeloid DCs prime for a TH2-type response74. However, it has also been suggested that the lymphoid DC subset induces tolerance, whereas the myeloid-derived DCs represent the migratory DCs involved in the activation of naive T cells74. The migration of myeloid DCs from the subepithelial dome of the Peyer's patch to the interfollicular T-cell region in response to microbial stimulation provides some support for this idea42. Exposure to anti-inflammatory mediators, such as PGE2 and IL-10 (which are prominent in the GALT14,15), have been shown to result in the differentiation of DCs that promote TH2 cells76,77. The induction of a TH2-type of response is sometimes equated with mucosal tolerance. However, recent work has shown that tolerance is not due simply to the deviation from a TH1 to a TH2-type of response (reviewed in Ref. 53). Moreover, the features that distinguish a 'tolerogenic' TH2 response from the 'immunogenic' TH2 response — induced by helminths and allergens (which is also typically localized to the mucosal surface) — are not yet clear.
Headquarters for regulatory T cells
There is evidence that DC subsets in the GALT have unique immune-inductive capabilities. High-level production of the TH2-type cytokines IL-4 and IL-10 by DCs in the Peyer's patch seems to polarize the mucosal immune response in an anti-inflammatory direction75,78. In particular, the IL-10 produced can be attributed to the myeloid DCs, which are typically localized in the subepithelial dome of the Peyer's patch. An unusual population of CD11b−CD8α− DCs predominates at mucosal surfaces and might have a special role at this site78. Moreover, only Peyer's patch DCs constitutively express messenger RNA for TGF-β42. These findings link cytokine production by Peyer's patch DCs to the generation of regulatory IL-10- (TR1)79,80 or TGF-β-(TH3)14 secreting T cells and might explain why these types of regulatory cells are preferentially generated in the GALT. Indeed, immature DCs (which fail to mature and secrete IL-12) might be central to the generation of these regulatory T cells81.
Functionally non-responsive anergic T cells can retain the ability to secrete immunosuppressive cytokines82. The rapid accumulation of data supporting the regulatory function of a subset of CD4+CD25+ T cells in both mice and humans has led to their recent 'certification' as professional suppressor T cells83. The induction of CD4+CD25+ suppressor cells by both oral and intravenous administration of soluble antigen has been documented, but their cytokine-secretion profile is not yet clear84. Subsets of regulatory/suppressor T cells that secrete TGF-β have long been implicated in oral tolerance52. Interestingly, CD4+CD25+ CD45RBlow+ regulatory T cells control intestinal inflammation in a mouse model of IBD85. These regulatory T cells are dependent on both TGF-β and signalling through CTLA-4, indicating a link between regulatory T cells and the induction of functional anergy that had not previously been appreciated85.
Concluding remarks
Recent work has enhanced our understanding of the ways in which immune responses are induced — or blocked —at the mucosal barrier through which most antigens enter. Much remains to be learned, especially with regard to the molecular mechanisms by which antigen presentation governs tolerance and immunity in the intestinal mucosa. Do specialized subpopulations of DCs control non-responsiveness to innocuous luminal antigens? If so, does their co-stimulatory molecule expression, cytokine secretion or trafficking have a role in regulating tolerance? The mechanism by which the innate immune system distinguishes commensal from pathogenic bacteria is a topic of great interest. Commensals bear PAMPs, which are capable of binding to TLRs, but don't elicit an immune response in healthy individuals. What signal(s) turn off the response to commensals (and what turns it on in inflammatory bowel disease)? Answers to these questions will provide information vital to the development of effective oral vaccines and the treatment of intestinal inflammatory and allergic diseases.
References
Madara, J. L. Regulation of movement of solutes across tight junctions. Annu. Rev. Physiol. 60, 143–159 (1998).
Ayabe, T. et al. Secretion of microbial α-defensins by intestinal Paneth cells in response to bacteria. Nature Immunol. 1, 113–118 (2000).
Macpherson, A. J. et al. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 288, 2222–2226 (2000).
Robinson, J. K., Blanchard, T. G., Levine, A. D., Emancipator, S. N. & Lamm, M. E. A mucosal IgA-mediated excretory immune system in vivo. J. Immunol. 166, 3688–3692 (2001).
Podolsky, D. K. Mucosal immunity and inflammation. V. Innate mechanisms of mucosal defense and repair: the best offense is a good defense. Am. J. Physiol. 277, G495–G499 (1999). | PubMed |
Kraehenbuhl, J.-P. & Neutra, M. R. Epithelial M cells: Differentiation and function. Annu. Rev. Cell. Dev. Biol. 16, 301–332 (2000).
Neutra, M. R. Current concepts in mucosal immunity. V. Role of M cells in transepithelial transport of antigens and pathogens to the mucosal immune system. Am. J. Physiol. 274, G785–G791 (1998).
Rescigno, M. et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nature Immunol. 2, 361–367 (2001).Describes a novel mechanism by which dendritic cells can sample both pathogenic and non-pathogenic luminal microbes.
Farstad, I. N., Carlsen, H., Morton, H. C. & Brandtzaeg, P. Immunoglobulin A cell distribution in the human small intestine: phenotypic and functional characteristics. Immunology 101, 354–363 (2000).
Kaetzel, C. S., Robinson, J. K., Chintalacharuvu, K. R., Vaerman, J.-P. & Lamm, M. E. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: a local defense function of IgA. Proc. Natl Acad. Sci. USA 88, 8796–8801 (1991).
Mostov, K. & Kaetzel, C. S. in Mucosal Immunology 2nd edn (eds Ogra, P. L. et al.) 181–211 (Academic Press, San Diego, 1999).
Cazac, B. B. & Roes, J. TGF-β receptors control B cell responsiveness and induction of IgA in vivo. Immunity 13, 443–451 (2000).
van Ginkel, F. W. et al. Partial IgA-deficiency with increased TH2-type cytokines in TGF-β1 knockout mice. J. Immunol. 163, 1951–1957 (1999).
Weiner, H. The mucosal milieu creates tolerogenic dendritic cells and TR1 and TH3 regulatory cells. Nature Immunol. 2, 671–672 (2001)
Newberry, R. D., Stenson, W. F. & Lorenz, R. G. Cyclooxygenase-2-dependent arachidonic acid metabolites are essential modulators of the intestinal immune response to dietary antigen. Nature Med. 5, 900–906 (1999).
Newberry, R. D., McDonough, J. S., Stenson, W. F. & Lorenz, R. G. Spontaneous and continuous cyclooxygenase-2-dependent prostaglandin E2 production by stromal cells in the murine small intestine lamina propria: directing the tone of the intestinal immune reponse. J. Immunol. 166, 4465–4472 (2001).
Suzuki, K. et al. Gut cryptopatches: direct evidence for extrathymic anatomical sites for intestinal T lymphopoiesis. Immunity 13, 691–702 (2000).
Laky, K. et al. Enterocyte expression of interleukin 7 induces development of γδ T cells and Peyer's patches. J. Exp. Med. 191, 1569–1580 (2000).
Masopust, D., Jiang, J., Shen, H. & Lefrancois, L. Direct analysis of the dynamics of the intestinal mucosa CD8 T cell response to systemic virus infection. J. Immunol. 166, 2348–2356 (2001).
Masopust, D., Vezys, V., Marzo, A. L. & Lefrancois, L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291, 2413–2417 (2001).
Reinhardt, R. L., Khoruts, A., Merica, R., Zell, T. & Jenkins, M. K. Visualizing the generation of memory CD4 T cells in the whole body. Nature 410, 101–105 (2001).References 20 and 21 show that memory T cells accumulate at mucosal surfaces, where they are ready to respond to the next antigenic challenge.
Sallusto, F., Lenig, D., Forster, R., Ripp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Wurbel, M.-A. et al. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur. J. Immunol. 30, 262–271 (2000).
Kunkel, E. J. et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-derived chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue specific chemokines as an organizing principle in regional immunity. J. Exp. Med. 192, 761–767 (2000).
Zabel, B. A. et al. Human G protein-coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J. Exp. Med. 190, 1241–1256 (1999).
Papadakis, K. A. et al. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J. Immunol. 165, 5069–5076 (2000).References 23–26 show that, in both mice and humans, tissue-specific expression of thymocyte-expressed chemokine (TECK) recruits CCR9+ lymphocytes to the small intestine.
McCracken, V. J. & Lorenz, R. G. The gastrointestinal ecosystem: a precarious alliance among epithelium, immunity and microbiota. Cell. Microbiol. 3, 1–11 (2001).
Medzhitov, R. & Janeway, C. A. Jr. Innate immunity: the virtues of a nonclonal system of recognition. Cell 91, 295–298 (1997).
Akira, S., Takeda, K. & Kaisho, T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nature Immunol. 2, 675–680 (2001).
Hopkins, S. A., Niedergang, F., Corthesy-Theulaz, E. & Kraehenbuhl, J.-P. A recombinant Salmonella typhimurium vaccine strain is taken up and survives within murine Peyer's patch dendritic cells. Cell. Microbiol. 2, 59–68 (2000).
Janeway, C. A. Jr. How the immune system works to protect the host from infection: a personal view. Proc. Natl Acad. Sci. USA 98, 7461–7468 (2001).
Beatty, W. L. & Sansonetti, P. J. Role of lipopolysaccharide in signaling to subepithelial polymorphonuclear leukocytes. Infect. Immun. 65, 4395–4404 (1997).
MacPherson, A. J. S. et al. IgA production without μ or δ chain expression in developing B cells. Nature Immunol. 2, 625–631 (2001).Zinkernagel and colleagues have described a previously unrecognized T-cell-independent pathway for B-cell differentiation to IgA-secreting plasma cells that predominates in the gut. This report shows that this pathway is present in mice with a block at the pro-B stage, indicating that it is characterized by the very early expression of the Igα heavy chain.
Gewirtz, A. T. & Madara, J. L. Periscopes up! Monitoring microbes in the intestine. Nature Immunol. 2, 288–290 (2001).
Ozinsky, A. et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation by Toll-like receptors. Proc. Natl Acad. Sci. USA 97, 13766–13771 (2000).
Cario, E. et al. Lipopolysaccharide activates distinct signalling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J. Immunol. 164, 966–972 (2000).
Elewaut, D. et al. NF-κB is a central regulator of the intestinal epithelial cell innate immune response induced by infection with enteroinvasive bacteria. J. Immunol. 163, 1457–1466 (1999).
Cario, E. & Podolsky, D. K. Differential alteration in intestinal epithelial cell expression of Toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect. Immun. 68, 7010–7017 (2000).
Colgan, S. P., Hershberg, R. M., Furuta, G. T. & Blumberg, R. S. Ligation of intestinal epithelial CD1d induces bioactive IL-10: critical role of the cytoplasmic tail in autocrine signalling. Proc. Natl Acad. Sci. USA 96, 13938–13943 (1999).
Philpott, D. J., Yamaoka, S., Israel, A. & Sansonetti, P. J. Invasive Shigella flexneri activates NF-κB through a lipopolysaccharide-dependent innate intracellular response that leads to IL-8 expression in epithelial cells. J. Immunol. 165, 903–914 (2000).
Cook, D. N. et al. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity 12, 495–503 (2000).
Iwasaki, A. & Kelsall, B. L. Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3α, MIP-3β, and secondary lympoid organ chemokine. J. Exp. Med. 191, 1381–1393 (2000).
Izadpanah, A., Dwinell, M. B., Eckmann, L., Varki, N. M. & Kagnoff, M. F. Regulated MIP-3α/CCL20 production by human intestinal epithelium: mechanism for modulating mucosal immunity. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G710–G719 (2001).References 41–43 show that CCR-6/MIP-3α is a second chemokine/receptor pair (the other is CCR9/CCL25) that is specific for the intestinal epithelium. The secretion of MIP-3α by the intestinal epithelium recruits CCR6+ dendritic cells into the intestinal mucosa.
Neish, A. et al. Prokaryotic regulation of epithelial responses by inhibition of IκB-α ubiquitination. Science 289, 1560–1563 (2000).Reports that binding of non-pathogenic bacteria to intestinal epithelial cells in vitro impairs transcription of pro-inflammatory cytokines by blocking the NF-κB/IκB signalling pathway.
Bhan, A., Mizoguchi, E., Smith, R. N. & Mizoguchi, A. Colitis in transgenic and knockout animals as models of human inflammatory bowel disease. Immunol. Rev. 169, 195–207 (1999).
Strober, W., Nakamura, K. & Kitani, A. The SAMP1/Yit mouse: another step closer to modeling human inflammatory disease. J. Clin. Invest. 107, 667–669 (2001).
Sartor, R. B. New therapeutic approaches to Crohn's disease. N. Engl. J. Med. 342, 1664–1666 (2000).
Hugot, J.-P. et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411, 599–603 (2001).
Ogura, Y. et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411, 603–606 (2001).
Hampe, J. et al. Association between insertion mutation in NOD2 gene and Crohn's disease in German and British populations. Lancet 357, 1925–1928 (2001).References 48–50 provide the first description of an 'IBD' gene. Mutations in a gene on chromosome 16 ( NOD2 ) are associated with the development of Crohn's disease in a subset of patients. Although the mechanism is not yet understood, the mutant form of NOD2 apparently alters signalling through NF-κB.
Beutler, B. Autoimmunity and apoptosis: the Crohn's connection. Immunity 15, 5–14 (2001).
Mowat, A. M. & Weiner, H. L. in Mucosal Immunology 2nd edn (eds Ogra, P. L. et al.) 587–618 (Academic Press, San Diego, 1999).
Nagler-Anderson, C. & Shi, H. N. Peripheral nonresponsiveness to orally administered soluble protein antigens. Crit. Rev. Immunol. 21, 121–132 (2001).
Shi, H. N., Grusby, M. J. & Nagler-Anderson, C. Orally induced peripheral nonresponsiveness is maintained in the absence of functional TH1 or TH2 cells. J. Immunol. 162, 5143–5148 (1999).
Kato, H., Fujihashi, K., Kato, R., Yuki, Y. & McGhee, J. R. Oral tolerance revisited: prior oral tolerization abrogates cholera toxin induced mucosal IgA responses. J. Immunol. 166, 3114–3121 (2001).
Borriello, F. et al. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity 6, 303–313 (1997).
Perez, V. L. et al. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity 6, 411–417 (1997).
Hershberg, R. M. and Mayer L. F. Antigen processing and presentation by intestinal epithelial cells — polarity and complexity. Immunol. Today 21, 123–128 (2000).
Nagler-Anderson, C., Terhorst, C., Bhan, A. K. & Podolsky, D. K. Mucosal antigen presentation and the control of tolerance and immunity. Trends Immunol. 22, 120–122 (2001).
Nagler-Anderson, C. Tolerance and immunity in the intestinal immune system. Crit. Rev. Immunol. 20, 103–120 (2000).
Sun, J., Dirden-Kramer, B., Ito, K., Ernst, P. B. & Van Houten, N. Antigen-specific T cell activation and proliferation during oral tolerance induction. J. Immunol. 162, 5868–5875 (1999).
Blanas, E., Davey, G. M., Carbone, F. R. & Heath, W. R. A bone marrow-derived APC in the gut-associated lymphoid tissue captures oral antigens and presents them to both CD4+ and CD8+ T cells. J. Immunol. 164, 2890–2896 (2000).
Shi, H. N., Liu, H. Y. & Nagler-Anderson, C. Enteric infection acts as an adjuvant for the response to a model food antigen. J. Immunol. 165, 6174–6182 (2000).
Whitacre, C. C., Gienapp, I. E., Orosz, C. G. and Bitar, D. M. Oral tolerance in experimental autoimmune encephalomyelitis. III. Evidence for clonal anergy. J. Immunol. 147, 2155–2163 (1991).
Chen, Y. et al. Peripheral deletion of antigen reactive T cells in oral tolerance. Nature 376, 177–179 (1995).
Greenwald, R. J., Boussiotis, V. A., Lorsbach, R. B., Abbas, A. K. & Sharpe, A. H. CTLA-4 regulates induction of anergy in vivo. Immunity 14, 145–155 (2001).
Huang, F.-P. et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 191, 435–443 (2000).Dendritic cell (DC) migration had been thought to require exposure to an inflammatory stimulus. This report shows that a subpopulation of DCs continuously tranports epithelial cell remnants (and presumably other antigens) from the intestinal mucosa to the T-cell areas of the mesenteric lymph node.
Vermaelen, K. Y., Carro-Muino, I., Lambrecht, B. N. & Pauwels, R. A. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J. Exp. Med. 193, 51–60 (2001).
Stumbles, P. A. et al. Regulation of dendritic cell recruitment into resting and inflamed airway epithelium: use of alternative chemokine receptors as a function of inducing stimulus. J. Immunol. 167, 228–234 (2001).
Sallusto, F. & Lanzavecchia, A. Mobilizing dendritic cells for tolerance, priming and chronic inflammation. J. Exp. Med. 189, 611–614 (1999).
Akbari, O., DeKruyff, R. H. & Umetsu, D. T. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nature Immunol. 2, 725–730 (2001).
Alpan, O., Rudomen, G. & Matzinger, P. The role of dendritic cells, B cells and M cells in gut oriented immune responses. J. Immunol. 166, 4843–4852 (2001).
Spahn, T. W. et al. Induction of oral tolerance to cellular immune responses in the absence of Peyer's patches. Eur. J. Immunol. 31, 1278–1287 (2001).
Pulendran, B., Maraskovsky, E., Banchereau, J. & Maliszewski, C. Modulating the immune response with dendritic cells and their growth factors. Trends Immunol. 22, 41–47 (2001).
Iwasaki, A. & Kelsall, B. L. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J. Exp. Med. 190, 229–239 (1999).
DeSmedt, T. et al. Effect of IL-10 on dendritic cell maturation and function. Eur. J. Immunol. 27, 1229–1235 (1997).
Kalinski, P., Hilkens, C. M., Snijders, A., Snijdewint, F. G. and Kapsenberg, M. L. IL-2-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive helper T cells. J. Immunol. 159, 28–35 (1997).
Iwasaki, A. & Kelsall, B. L. Unique functions of CD11b+, CD8α+, and double-negative Peyer's patch dendritic cells. J. Immunol. 166, 4884–4890 (2001).
Groux, H. et al. A CD4+ T cell subset inhibits antigen-specific T cell responses and prevents colitis. Nature 389, 737–742 (1997).
Cottrez, F., Hurst, S. D., Coffman, R. L. & Groux, H. T regulatory cells 1 inhibit a TH2-specific response in vivo. J. Immunol. 165, 4848–4853 (2000).
Roncarolo, M. G., Levings, M. K. & Traversari, C. Differentiation of T regulatory cells by immature dendritic cells. J. Exp. Med. 193, F5–F9 (2001).
Jooss, K., Gjata, B., Danos, O., Boehmer, H. V. & Sarukhan, A. Regulatory function of in vivo anergized CD4+ T cells. Proc. Natl Acad. Sci. USA 98, 8738–8743 (2001).
Shevach, E. M. Certified professionals: CD4+CD25+ suppressor T cells. J. Exp. Med. 193, F41–F45 (2001).
Thorstenson, K. M. & Khoruts, A. Generation of anergic and potentially immunoregulatory CD25+CD4+T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J. Immunol. 167, 188–195 (2001).
Read, S., Malmstrom, V. & Powrie, F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192, 295–302 (2000).
Neutra, M. R., Frey, A. & Kraehenbuhl, J. P. Epithelial M cells: gateways for mucosal infection and immunization. Cell 86, 345–348 (1996).
Mantis, N. J., Frey, A. & Neutra, M. R. Accessibility of glycolipid and oligosaccharide epitopes on rabbit villus and follicle-associated epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 278, G915–G923 (2000).
Clark, M., Hirst, B. H. & Jepson, M. A. M-cell surface β1 integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer's patch M cells. Infect. Immun. 66, 1237–1243 (1998).
Grutzkau, A., Hanski, C., Hahn, H. & Riecken, E. O. Involvement of M cells in the bacterial invasion of Peyer's patches: a common mechanism shared by Yersinia enterocolitis and other enteroinvasive bacteria. Gut 31, 1011–1015 (1990).
Jones, B. D., Ghori, N. & Falkow, S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J. Exp. Med. 180, 15–23 (1994).
Sansonetti, P. J., Arondel, J., Cantey, J. R., Prevost, M. C. & Huerre, M. Infection of rabbit Peyer's patches by Shigella flexneri: effect of adhesive or invasive bacterial phenotype on follicle-associated epithelium. Infect. Immun. 64, 2752–2764 (1996).
Owen, R. L., Pierce, N. F., Apple, R. T. & Cray, W. C. C. Jr. M cell transport of Vibrio cholerae from the intestinal lumen into Peyer's patches: a mechanism for antigen sampling and for microbial transepithelial migration. J. Infect. Dis. 153, 1108–1118 (1986).
Inman, L. R. & Cantey, J. R. Specific adherence of Escherichia coli (strain RDCE-1) to membranous (M) cells of the Peyer's patch in Escherichia coli diarrhea in the rabbit. J. Clin. Invest. 71, 1–8 (1983).
Amerongen, H. M., Wilson, G. A. R., Fields, B. N. & Neutra, M. R. Proteolytic processing of reovirus is required for adherence to intestinal M cells. J. Virol. 68, 8428–8432 (1994).
Roberts, M., Li, J., Bacon, A. & Chatfield, S. Oral vaccination against Tetanus: Comparison of the immunogenicities of Salmonella strains expressing fragment C from the nirB and htrA promoters. Infect. Immun. 66, 3080–3087 (1998).
McCluskie, M. J., Weeratna, R. D., Clements, J. D. & Davis, H. L. Mucosal immunization of mice using CpG and/or mutants of the heat-labile enterotoxin of Escherichia coli as adjuvants. Vaccine 19, 3759–3768 (2001).
Millar, D. G., Hirst, T. R. & Snider, D. P. Escherichia coli heat-labile enterotoxin B is a more potent mucosal adjuvant than its closely related homogue, the B subunit of cholera toxin. Infect. Immun. 69, 3476–3482 (2001).
Staats, H. F. & Ennis, F. A. Jr. IL-1 is an effective adjuvant for mucosal and systemic immune responses when coadministered with protein immunogens. J. Immunol. 162, 6141–6147 (1999).
Acknowledgements
This work was supported by National Institutes of Health and by the Center for the Study of Inflammatory Bowel Disease at Massachusetts General Hospital and the Clinical Nutrition Research Center at Harvard. I thank the members of my laboratory for their contributions to both the work cited and the writing of this manuscript, particularly: H.N. Shi, E. Melendro, M. Bashir, D. Smith, P. Andersen and O. Iweala. Thanks are also extended to B. McCormick, B. Cherayil, A. Bhan and D. Podolsky for critical review of the manuscript.
Author information
Authors and Affiliations
Glossary
- TIGHT JUNCTIONS
-
A ring of proteins that seals apical epithelium; includes the integral membrane proteins occludin and claudin, in association with cytoplasmic zonula occludins proteins.
- DEFENSINS
-
Anti-microbial peptides secreted by Paneth cells in the villus crypt.
- TREFOIL PEPTIDES
-
Three small proteins, secreted by goblet cells, which function in epithelial protection and repair5.
- SECRETORY COMPONENT
-
Fragment of the polymeric immunoglobulin receptor receptor that is left attached to secretory IgA after its transport to the apical surface of epithelial cells.
- TRANSFORMING GROWTH FACTOR-β
-
(TGF-β). A pleotropic anti-inflammatory cytokine produced by activated T cells and mononuclear phagocytes as well as other cell types. The effects of TGF-β are mainly anti-proliferative. It antagonizes the actions of pro-inflammatory cytokines by inhibiting both macrophage activation and T-cell proliferation and differentiation.
- ARACHIDONIC ACID METABOLITES
-
A family of lipid mediators with diverse biological activities. The products of the lipooxygenase and cyclooxygenase (COX) pathways, leukotrienes and prostaglandins, respectively, are important mediators of the allergic inflammatory response. Prostaglandin E2 has immunomodulatory effects in the small intestinal lamina propria, where it is secreted at high levels due to the constitutive production of COX2.
- CRYPTOPATCHES
-
Clusters of c-kit+IL-7R+Thy1+ T-cell progenitors found in the crypt lamina propria of both small and large intestinal villi.
- VASCULAR ADDRESSINS
-
Mucin-like molecules, expressed on endothelial cells, to which leukocyte-adhesion molecules (homing receptors) bind. Vascular addressins have a central role in guiding the selective homing of lymphocytes to various body sites. In the mucosa, the mucosal addressin cell-adhesion molecule-1 (MadCAM-1) binds to both L-selectin (CD62L) and the integrin α4β7.
- NF-κB/IκB PATHWAY
-
In resting lymphocytes, the transcription factor NF-κB forms a complex with IκB in the cytoplasm. Engagement of Toll receptors by microbes activates a cascade that leads ultimately to activation of the NF-κB kinase, NIK, and the formation of the dimer Iκk. Iκk phosphorylates IκB, resulting in its dissociation from NF-κB. NF-κB then enters the nucleus where it binds to (and activates) the promoters of various pro-inflammatory cytokines genes.
- CROHN'S DISEASE
-
One of two idiopathic inflammatory bowel diseases that is characterized by chronic intestinal inflammation. The inflammatory lesions of Crohn's Disease can occur in any part of the gastrointestinal tract. By contrast, ulcerative colitis is typically confined to the colon.
Rights and permissions
About this article
Cite this article
Nagler-Anderson, C. Man the barrier! strategic defences in the intestinal mucosa. Nat Rev Immunol 1, 59–67 (2001). https://doi.org/10.1038/35095573
Issue Date:
DOI: https://doi.org/10.1038/35095573