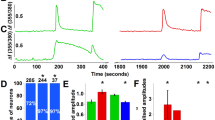
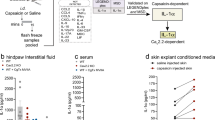
Abstract
Tissue injury generates endogenous factors that heighten our sense of pain by increasing the response of sensory nerve endings to noxious stimuli1,2. Bradykinin and nerve growth factor (NGF) are two such pro-algesic agents that activate G-protein-coupled (BK2) and tyrosine kinase (TrkA) receptors, respectively, to stimulate phospholipase C (PLC) signalling pathways in primary afferent neurons3,4. How these actions produce sensitization to physical or chemical stimuli has not been elucidated at the molecular level. Here, we show that bradykinin- or NGF-mediated potentiation of thermal sensitivity in vivo requires expression of VR1, a heat-activated ion channel on sensory neurons. Diminution of plasma membrane phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) levels through antibody sequestration or PLC-mediated hydrolysis mimics the potentiating effects of bradykinin or NGF at the cellular level. Moreover, recruitment of PLC-γ to TrkA is essential for NGF-mediated potentiation of channel activity, and biochemical studies suggest that VR1 associates with this complex. These studies delineate a biochemical mechanism through which bradykinin and NGF produce hypersensitivity and might explain how the activation of PLC signalling systems regulates other members of the TRP channel family.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
McMahon, S. B. & Bennett, D. L. H. in Textbook of Pain (eds Wall, P. D. & Melzack, R.) 105–128 (Harcourt, London, 1999).
Bevan, S. in Textbook of Pain (eds Wall, P. D. & Melzack, R.) 85–103 (Harcourt, London, 1999).
Ganju, P., O'Bryan, J. P., Der, C., Winter, J. & James, I. F. Differential regulation of SHC proteins by nerve growth factor in sensory neurons and PC12 cells. Eur. J. Neurosci. 10, 1995–2008 (1998).
Burgess, G. M., Mullaney, I., McNeill, M., Dunn, P. M. & Rang, H. P. Second messengers involved in the mechanism of action of bradykinin in sensory neurons in culture. J. Neurosci. 9, 3314–3325 (1989).
Woolf, C. J. & Salter, M. W. Neuronal plasticity: increasing the gain in pain. Science 288, 1765–1769 (2000).
Shu, X. Q. & Mendell, L. M. Neurotrophins and hyperalgesia. Proc. Natl Acad. Sci. USA 96, 7693–7696 (1999).
Nicholas, R. S., Winter, J., Wren, P., Bergmann, R. & Woolf, C. J. Peripheral inflammation increases the capsaicin sensitivity of dorsal root ganglion neurons in a nerve growth factor-dependent manner. Neuroscience 91, 1425–1433 (1999).
Koltzenburg, M., Bennett, D. L., Shelton, D. L. & McMahon, S. B. Neutralization of endogenous NGF prevents the sensitization of nociceptors supplying inflamed skin. Eur. J. Neurosci. 11, 1698–1704 (1999).
Woolf, C. J. Phenotypic modification of primary sensory neurons: the role of nerve growth factor in the production of persistent pain. Phil. Trans. R. Soc. Lond. B 351, 441–448 (1996).
Koltzenburg, M. The changing sensitivity in the life of the nociceptor. Pain 6 (Suppl.), S93–S102 (1999).
Caterina, M. J. et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 (1997).
Tominaga, M. et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21, 531–543 (1998).
Caterina, M. J. et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288, 306–313 (2000).
Davis, J. B. et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 405, 183–187 (2000).
Jordt, S. E., Tominaga, M. & Julius, D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc. Natl Acad. Sci. USA 97, 8134–8139 (2000).
Harteneck, C., Plant, T. D. & Schultz, G. From worm to man: three subfamilies of TRP channels. Trends Neurosci. 23, 159–166 (2000).
Shu, X. & Mendell, L. M. Nerve growth factor acutely sensitizes the response of adult rat sensory neurons to capsaicin. Neurosci. Lett. 274, 159–162 (1999).
Bergmann, I., Reiter, R., Toyka, K. V. & Koltzenburg, M. Nerve growth factor evokes hyperalgesia in mice lacking the low-affinity neurotrophin receptor p75. Neurosci. Lett. 255, 87–90 (1998).
Stephens, R. M. et al. Trk receptors use redundant signal transduction pathways involving SHC and PLC-γ1 to mediate NGF responses. Neuron 12, 691–705 (1994).
Cesare, P., Dekker, L. V., Sardini, A., Parker, P. J. & McNaughton, P. A. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron 23, 617–624 (1999).
Premkumar, L. S. & Ahern, G. P. Induction of vanilloid receptor channel activity by protein kinase C. Nature 408, 985–990 (2000).
Womack, K. B. et al. Do phosphatidylinositides modulate vertebrate phototransduction? J. Neurosci. 20, 2792–2799 (2000).
Huang, C. L., Feng, S. & Hilgemann, D. W. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature 391, 803–806 (1998).
Zhang, H., He, C., Yan, X., Mirshahi, T. & Logothetis, D. E. Activation of inwardly rectifying K+ channels by distinct PtdIns(4,5)P2 interactions. Nature Cell Biol. 1, 183–188 (1999).
Li, H. S., Xu, X. Z. & Montell, C. Activation of a TRPC3-dependent cation current through the neurotrophin BDNF. Neuron 24, 261–273 (1999).
Estacion, M., Sinkins, W. G. & Schilling, W. P. Regulation of Drosophila transient receptor potential-like (TrpL) channels by phospholipase C-dependent mechanisms. J. Physiol. 530, 1–19 (2001).
Zygmunt, P. M. et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400, 452–457 (1999).
Hofmann, T. et al. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397, 259–263 (1999).
Chevesich, J., Kreuz, A. J. & Montell, C. Requirement for the PDZ domain protein, INAD, for localization of the TRP store-operated channel to a signaling complex. Neuron 18, 95–105 (1997).
Scott, K. & Zuker, C. S. Assembly of the Drosophila phototransduction cascade into a signalling complex shapes elementary responses. Nature 395, 805–808 (1998).
Acknowledgements
We are grateful to W. Neuhausser for providing dissociated DRG neurons, W. Mobley for 2.5 S NGF, D. Shelton for advice regarding NGF administration and J. Poblete and K. Simpson for technical assistance. We thank members of our laboratories for many helpful suggestions and constructive criticism. This work was supported by a NSF predoctoral fellowship (E.P.), NIH predoctoral neuroscience training grant (S.S.), a German Academy of Natural Scientists Leopoldina postdoctoral fellowship (S.-E.J.) and by the NIH (M.V.C., A.I.B. and D.J.).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figure 1
(A) In transfected HEK cells, BK (20 nM) elicits a strongly rectifying VR1 basal current that is inhibited by 5 mM capsazepine, as shown by the holding current trace (left) and the ramp traces (right). (B) PIP2 Ab-induced VR1 basal current (-60 mV) is suppressed by 3 mM capsazepine; representative ramp traces from specified time points (* and **) are shown at right. (PDF 26 kb)
Supplementary Figure 2
[3H]-RTX binding to membranes from VR1-transfected HEK293 cells is displaced by TPA. Data are plotted as percentage of specific [3H]-RTX binding. IC50 for TPA = 256 ± 8 nM (average of four independent experiments; each point in duplicate). Note that phorbol-12,13-dibutyrate (PDBu) did not affect [3H]-RTX binding. Data were fit to the Hill equation. (DOC 33 kb)
Supplementary Figure 3
VR1 associates with wild type TrkA and TrkA mutants. HEK cells were transfected with equal amounts of VR1 and TrkA or TrkA mutant cDNAs. Immune complexes (above) were formed and analyzed as described in text. Western blots (below) of 40 mg total soluble HEK protein indicate equal expression levels of VR1 and Trks among samples. (PDF 21 kb)
Supplementary Figure 4
Effects of EGFR activation on VR1 currents. Two-electrode voltage clamp analysis was performed as described in text. Representative traces are shown above, and quantitation of 5 VR1 injected and 8 VR1/EGFR injected oocytes is shown below as SEM, p < 0.005. Note calcium activated chloride current (denoted with star) indicative of EGFR stimulation and consequent PLC activation. (PDF 26 kb)
Rights and permissions
About this article
Cite this article
Chuang, Hh., Prescott, E., Kong, H. et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 411, 957–962 (2001). https://doi.org/10.1038/35082088
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35082088
This article is cited by
-
Anatomical differences in nociceptor neurons sensitivity
Bioelectronic Medicine (2022)
-
FABP5 deletion in nociceptors augments endocannabinoid signaling and suppresses TRPV1 sensitization and inflammatory pain
Scientific Reports (2022)
-
Critical contributions of pre-S1 shoulder and distal TRP box in DAG-activated TRPC6 channel by PIP2 regulation
Scientific Reports (2022)
-
Targeting TRPV1-mediated autophagy attenuates nitrogen mustard-induced dermal toxicity
Signal Transduction and Targeted Therapy (2021)
-
Lifting the veil on the keratinocyte contribution to cutaneous nociception
Protein & Cell (2020)