Abstract
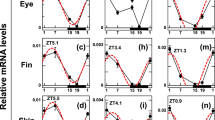
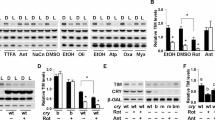
Cryptochromes are flavin/pterin-containing proteins that are involved in circadian clock function in Drosophila and mice. In mice, the cryptochromes Cry1 and Cry2 are integral components of the circadian oscillator within the brain1,2,3,4,5,6 and contribute to circadian photoreception in the retina7. In Drosophila, cryptochrome (CRY) acts as a photoreceptor that mediates light input to circadian oscillators in both brain and peripheral tissue8,9,10,11,12. A Drosophila cry mutant, cryb, leaves circadian oscillator function intact in central circadian pacemaker neurons but renders peripheral circadian oscillators largely arrhythmic. Although this arrhythmicity could be caused by a loss of light entrainment, it is also consistent with a role for CRY in the oscillator. A peripheral oscillator drives circadian olfactory responses in Drosophila antennae13. Here we show that CRY contributes to oscillator function and physiological output rhythms in the antenna during and after entrainment to light–dark cycles and after photic input is eliminated by entraining flies to temperature cycles. These results demonstrate a photoreceptor-independent role for CRY in the periphery and imply fundamental differences between central and peripheral oscillator mechanisms in Drosophila.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
van der Horst, G. T. J. et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398, 627–630 (1999).
Vitaterna, M. H. et al. Differential regulation of mammalian Period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc. Natl Acad. Sci. USA 96, 12114–12119 (1999).
Kume, K. et al. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98, 193–205 (1999).
Griffin, E. A. J., Staknis, D. & Weitz, C. J. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science 286, 768–771 (1999).
Okamura, H. et al. Photic induction of mPer1 and mPer2 in Cry-deficient mice lacking a biological clock. Science 286, 2531–2534 (1999).
Shearman, L. P. et al. Interacting molecular loops in the mammalian circadian clock. Science 288, 1013–1019 (2000).
Selby, C. P., Thompson, C., Schmitz, T. M., Van Gelder, R. N. & Sancar, A. Functional redundancy of cryptochromes and classical photoreceptors for nonvisual ocular photoreception in mice. Proc. Natl Acad. Sci. USA 97, 14697–14702 (2000).
Stanewsky, R. et al. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell 95, 681–692 (1998).
Emery, P., So, W. V., Kaneko, M., Hall, J. C. & Rosbash, M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell 95, 669–679 (1998).
Ishikawa, T. et al. DCRY is a Drosophila photoreceptor protein implicated in light entrainment of circadian rhythm. Genes Cells 4, 57–65 (1999).
Emery, P. et al. CRY is a deep brain circadian photoreceptor. Neuron 26, 493–504 (2000).
Emery, P., Stanewsky, R., Hall, J. C. & Rosbash, M. A unique circadian-rhythm photoreceptor. Nature 404, 456–457 (2000).
Krishnan, B., Dryer, S. E. & Hardin, P. E. Circadian rhythms in olfactory responses of Drosophila melanogaster. Nature 400, 375–378 (1999).
Plautz, J. D., Kaneko, M., Hall, J. C. & Kay, S. A. Independent photoreceptive circadian clocks throughout Drosophila. Science 278, 1632–1635 (1997).
Plautz, J. D. et al. Quantitative analysis of Drosophila period gene transcription in living animals. J. Biol. Rhythms 12, 204–217 (1997).
Stanewsky, R., Jamison, C. F., Plautz, J. D., Kay, S. A. & Hall, J. C. Multiple circadian-regulated elements contribute to cycling period gene expression in Drosophila. EMBO J. 16, 5006–5018 (1997).
Wheeler, D. A., Hamblen-Coyle, M. J., Dushay, M. S. & Hall, J. C. Behavior in light-dark cycles of Drosophila mutants that are arrythmic, blind or both. J. Biol. Rhythms 8, 67–94 (1993).
Ceriani, M. F. et al. Light-dependent sequestration of TIMELESS by CRYPTOCHROME. Science 285, 553–556 (1999).
Field, M. D. et al. Analysis of clock proteins in mouse SCN demonstrates phylogenetic divergence of the circadian clockwork and resetting mechanisms. Neuron 25, 437–447 (2000).
Power, J., Ringo, J. & Dowse, H. The role of light in the initiation of circadian activity rhythms of adult Drosophila melanogaster. J. Neurogenet. 9, 227–238 (1995).
Brandes, C. et al. Novel features of Drosophila per transcription revealed by real-time luciferase reporting. Neuron 16, 687–692 (1996).
Chatfield, C. The Analysis of Time Series (Chapman and Hall/CRC, London, 1999).
Dowse, H. B. & Ringo, J. M. The search for hidden periodicities in biological time series revisited. J. Theor. Biol. 139, 487–515 (1989).
Dowse, H. et al. A congenital heart defect in Drosophila caused by an action-potential mutation. J. Neurogenet. 10, 153–168 (1995).
Johnson, E., Ringo, J., Bray, N. & Dowse, H. Genetic and pharmacological identification of ion channels central to the cardiac pacemaker. J. Neurogenet. 12, 1–24 (1998).
Dowse, H. B. & Ringo, J. M. Comparisons between “periodograms” and spectral analysis: apples are apples after all. J. Theor. Biol. 148, 139–144 (1991).
Acknowledgements
We thank R. Stanewsky for comments on the manuscript and additional tim–luc lines. This work was supported by US NIH grants to J.C.H., P.E.H. and S.E.D.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Rights and permissions
About this article
Cite this article
Krishnan, B., Levine, J., Lynch, M. et al. A new role for cryptochrome in a Drosophila circadian oscillator. Nature 411, 313–317 (2001). https://doi.org/10.1038/35077094
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35077094