Abstract
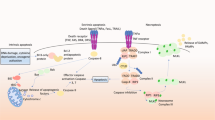
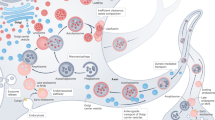
Neuronal death underlies the symptoms of many human neurological disorders, including Alzheimer's, Parkinson's and Huntington's diseases, stroke, and amyotrophic lateral sclerosis. The identification of specific genetic and environmental factors responsible for these diseases has bolstered evidence for a shared pathway of neuronal death — apoptosis — involving oxidative stress, perturbed calcium homeostasis, mitochondrial dysfunction and activation of cysteine proteases called caspases. These death cascades are counteracted by survival signals, which suppress oxyradicals and stabilize calcium homeostasis and mitochondrial function. With the identification of mechanisms that either promote or prevent neuronal apoptosis come new approaches for preventing and treating neurodegenerative disorders.
Key Points
-
Neuronal death underlies the symptoms of many human neurological disorders, including Alzheimer's, Parkinson's and Huntington's diseases, stroke, and amyotrophic lateral sclerosis.
-
Many signals can initiate apoptosis in neurons, including lack of neurotrophic factor support, overactivation of glutamate receptors (leading to calcium influx), increased oxidative stress and metabolic stress.
-
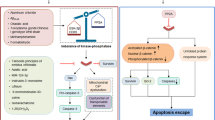
Mitochondrial changes are pivotal in the cell death decision in many cases. Mitochondria in cells undergoing apoptosis show increased oxyradical production, opening of pores in their membranes and release of cytochrome c.
-
The Bcl-2 family of proteins includes both anti-apoptotic (for example, Bcl-2) and pro-apoptotic (for example, Bax) members.
-
Overexpression of Bcl-2 in cell cultures and in transgenic mice increases resistance of neurons to death induced by excitotoxic, metabolic and oxidative insults. Conversely, neurons lacking Bax are protected against apoptosis.
-
Further mechanisms that can regulate the early stages of apoptosis in neurons involve caspases (evolutionarily conserved cysteine proteases central to apoptosis of many cell types), Par-4 and telomerase.
-
Neurotrophic factors can protect neurons against apoptosis by activating receptors linked to production of cell survival-promoting proteins (such as antioxidant enzymes, Bcl-2 family members and proteins involved in calcium homeostasis) through kinase cascades.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wyllie, A. H. Apoptosis and carcinogenesis. Eur. J. Cell Biol. 73 , 189–197 (1997). Provides a historical overview of the morphological and biochemical features of apoptosis.
Oppenheim, R. W. Cell death during development of the nervous system. Annu. Rev. Neurosci. 14, 453–501 ( 1991).
Mattson, M. P. & Lindvall, O. in The Aging Brain Vol. 2 (eds Mattson, M. P. & Geddes, J. W.) 299– 345 (JAI Press, Greenwich, Connecticut, 1997).
McKay, S. E., Purcell, A. L. & Carew, T. J. Regulation of synaptic function by neurotrophic factors in vertebrates and invertebrates: implications for development and learning . Learn. Mem. 6, 193–215 (1999).
Ankarcrona, M. et al. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron 15, 961–973 (1995).
Glazner, G. W., Chan, S. L., Lu, C. & Mattson, M. P. Caspase-mediated degradation of AMPA receptor subunits: a mechanism for preventing excitotoxic necrosis and ensuring apoptosis. J. Neurosci. 20, 3641–3649 (2000).
Choi, D. W. Excitotoxic cell death. J. Neurobiol. 23, 1261–1276 (1992).
Wong, P. C., Rothstein, J. D. & Price, D. L. The genetic and molecular mechanisms of motor neuron disease. Curr. Opin. Neurobiol. 8, 791– 799 (1998).
Sastry, P. S. & Rao, K. S. Apoptosis and the nervous system . J. Neurochem. 74, 1–20 (2000).
Mattson, M. P. Modification of ion homeostasis by lipid peroxidation: roles in neuronal degeneration and adaptive plasticity. Trends Neurosci. 21, 53–57 (1998).
Beal, M. F. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann. Neurol. 38, 357–366 (1995).
Duan, W., Zhang, Z., Gash, D. M. & Mattson, M. P. Participation of prostate apoptosis response-4 in degeneration of dopaminergic neurons in models of Parkinson's disease. Ann. Neurol. 46, 587–597 (1999).
Kroemer, G., Dallaporta, B. & Resche-Rigon, M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu. Rev. Physiol. 60, 619 –642 (1998).Detailed review of the role of mitochondria in apoptosis.
Matsumoto, S., Friberg, H., Ferrand-Drake, M. & Wieloch, T. Blockade of the mitochondrial permeability transition pore diminishes infarct size in the rat after transient middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 19, 736– 741 (1999).
Pellegrini, M. & Strasser, A. A portrait of the Bcl-2 protein family: life, death, and the whole picture. J. Clin. Immunol. 19, 365–377 (1999).
Martinou, J. C. et al. Overexpression of Bcl-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron 13, 1017–1030 ( 1994).Direct evidence that Bcl-2 can prevent death of neurons in vivo during development and in an experimental stroke model.
Guo, Q. et al. Alzheimer's presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid β-peptide: involvement of calcium and oxyradicals. J. Neurosci. 17, 4212–4222 (1997).
White, F. A., Keller-Peck, C. R., Knudson, C. M., Korsmeyer, S. J. & Snider, W. D. Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. J. Neurosci. 18, 1428–1439 (1998).Direct evidence that the pro-apoptotic protein Bax is essential in natural cell death during development of the nervous system.
Green, D. R. & Reed, J. C. Mitochondria and apoptosis. Science 28, 1309–1312 ( 1998).
Chan, S. L. & Mattson, M. P. Caspase and calpain substrates: roles in synaptic plasticity and cell death. J. Neurosci. Res. 58, 167–190 ( 1999).
Raoul, C., Pettmann, B. & Henderson, C. E. Active killing of neurons during development and following stress: a role for p75(NTR) and Fas? Curr. Opin. Neurobiol. 10, 111–117 ( 2000).
Nagata, S. Apoptotic DNA fragmentation. Exp. Cell Res. 256, 12–18 (2000).
Guo, Q. et al. Par-4 is a mediator of neuronal degeneration associated with the pathogenesis of Alzheimer disease. Nature Med. 4, 957–962 (1998).Initial description and characterization of the involvement of Par-4 in neuronal apoptosis in experimental models of developmental cell death and Alzheimer's disease.
Mattson, M. P., Duan, W., Chan, S. L. & Camandola, S. Par-4: an emerging pivotal player in neuronal apoptosis and neurodegenerative disorders. J. Mol. Neurosci. 13, 17–30 (1999).
Liu, J. P. Studies of the molecular mechanisms in the regulation of telomerase activity . FASEB J. 13, 2091–2104 (1999).
Fu, W., Killen, M., Pandita, T. & Mattson, M. P. The catalytic subunit of telomerase is expressed in developing brain neurons and serves a cell survival-promoting function. J. Mol. Neurosci. 14, 3–15 (2000).
Fu, W., Begley, J. G., Killen, M. W. & Mattson, M. P. Anti-apoptotic role of telomerase in pheochromocytoma cells. J. Biol. Chem. 274, 7264–7271 (1999).
Tamatani, M., Ogawa, S., Nunez, G. & Tohyama, M. Growth factors prevent changes in Bcl-2 and Bax expression and neuronal apoptosis induced by nitric oxide. Cell Death Differ. 5, 911 –919 (1998).
Hagg, T. & Varon, S. Ciliary neurotrophic factor prevents degeneration of adult rat substantia nigra dopaminergic neurons in vivo . Proc. Natl Acad. Sci. USA 90, 6315 –6319 (1993).
Middleton, G. et al. Cytokine-induced nuclear factor kappa B activation promotes the survival of developing neurons. J. Cell Biol. 148 , 325–332 (2000).
Mattson, M. P. & Camandola, S. NF-κB in neurodegenerative disorders. J. Clin. Invest. 61 , 134–139 (2000).
Yu, Z. F., Zhou, D., Bruce-Keller, A. J., Kindy, M. S. & Mattson, M. P. Lack of the p50 subunit of NF-κB increases the vulnerability of hippocampal neurons to excitotoxic injury. J. Neurosci. 19, 8856–8865 (1999).
Aschner, M., Allen, J. W., Kimelberg, H. K., LoPachin, R. M. & Streit, W. J. Glial cells in neurotoxicity development. Annu. Rev. Pharmacol. Toxicol. 39, 151–173 (1999).
Yu, Z. F. & Mattson, M. P. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J. Neurosci. Res. 15, 830–839 (1999).
Lee, J., Duan, W., Long, J. M., Ingram, D. K. & Mattson, M. P. Dietary restriction increases survival of newly-generated neural cells and induces BDNF expression in the dentate gyrus of rats. J. Mol. Neurosci. (in the press).
Berridge, M. J., Lipp, P. & Bootman, M. D. The versatility and universality of calcium signalling . Nature Rev. Mol. Cell Biol. 1, 11– 21 (2000). [ Contents page]
Yano, S., Tokumitsu, H. & Soderling, T. R. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature 396, 584–587 (1998).
Hu, S. C., Chrivia, J. & Ghosh, A. Regulation of CBP-mediated transcription by neuronal calcium signaling. Neuron 22, 799– 808 (1999).
Furukawa, K. et al. The actin-severing protein gelsolin modulates calcium channel and NMDA receptor activities and vulnerability to excitotoxicity in hippocampal neurons. J. Neurosci. 17, 8178– 8186 (1997).
Furukawa, K., Barger, S. W., Blalock, E. M. & Mattson, M. P. Activation of K+ channels and suppression of neuronal activity by secreted β-amyloid-precursor protein. Nature 379, 74–78 (1996).
Guenette, S. Y, & Tanzi, R. E. Progress toward valid transgenic mouse models for Alzheimer's disease. Neurobiol. Aging 20, 201–211 ( 1999).
Sathasivam, K. et al. Transgenic models of Huntington's disease. Philos. Trans. R. Soc. Lond. B 354, 963–969 (1999).
Borchelt, D. R., Wong, P. C., Sisodia, S. S. & Price, D. L. Transgenic mouse models of Alzheimer's disease and amyotrophic lateral sclerosis . Brain Pathol. 8, 735– 757 (1998).
Cummings, J. L., Vinters, H. V., Cole, G. M. & Khachaturian, Z. S. Alzheimer's disease: etiologies, pathophysiology, cognitive reserve, and treatment opportunities. Neurology 51, S2– S17 (1998).
Haass, C. & De Strooper, B. The presenilins in Alzheimer's disease — proteolysis holds the key. Science 286, 916–919 (1999).
Su, J. H., Anderson, A. J., Cummings, B. & Cotman, C. W. Immunocytochemical evidence for apoptosis in Alzheimer's disease. Neuroreport 5, 2529–2533 (1994).
Masliah, E., Mallory, M., Alford, M., Tanaka, S. & Hansen, L. A. Caspase dependent DNA fragmentation might be associated with excitotoxicity in Alzheimer disease. J. Neuropathol. Exp. Neurol. 57, 1041–1052 ( 1998).
Suzuki, T. et al. Molecular cloning of a novel apoptosis-related gene, human Nap1 (NCKAP1), and its possible relation to Alzheimer disease. Genomics 63, 246–254 ( 2000).
Loo, D., Copani, A., Pike, C., Whittemore, E., Walencewicz, A. & Cotman, C. W. Apoptosis is induced by β-amyloid in cultured central nervous system neurons. Proc. Natl Acad. Sci. USA 90, 7951 –7955 (1993).
Weidemann, A. et al. Proteolytic processing of the Alzheimer's disease amyloid precursor protein within its cytoplasmic domain by caspase-like proteases . J. Biol. Chem. 274, 5823– 5829 (1999).
Chen, Y., McPhie, D. L., Hirschberg, J. & Neve, R. L. The amyloid precursor protein-binding protein APP-BP1 drives the cell cycle through the S–M checkpoint and causes apoptosis in neurons. J. Biol. Chem. 275, 8929–8935 (2000).
Lu, D. C. et al. A second cytotoxic proteolytic peptide derived from amyloid precursor protein. Nature Med. 6, 397– 404 (2000).
Guo, Q. et al. Neurotrophic factors (activity-dependent neurotrophic factor (ADNF) and basic fibroblast growth factor (bFGF)) interrupt excitotoxic neurodegenerative cascades promoted by a presenilin-1 mutation. Proc. Natl Acad. Sci. USA 96, 4125–4130 ( 1999).Uses knock-in mice to identify the gain-of-function action of mutant presenilin-1 as a pro-apoptotic activity that results from perturbed cellular calcium homeostasis and can be suppressed by neurotrophic factors.
Mattson, M. P. et al. Calcium signaling in the ER: its role in neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 23 , 222–229 (2000).
Vito, P., Lacana, E. & d'Adamio, L. Interfering with apoptosis: Ca2+-binding protein ALG-2 and Alzheimer's disease gene ALG-3. Science 271, 521–525 ( 1996).
Jenner, P. & Olanow, C. W. Understanding cell death in Parkinson's disease. Ann. Neurol. 44, S72– S84 (1998).
Polymeropoulos, M. H. Autosomal dominant Parkinson's disease and alpha-synuclein. Ann. Neurol. 44, S63–S64 ( 1998).
Klevenyi, P. et al. Transgenic mice expressing a dominant negative mutant interleukin-1β converting enzyme show resistance to MPTP neurotoxicity. Neuroreport 10, 635–638 ( 1999).
Gash, D. M. et al. Functional recovery in parkinsonian monkeys treated with GDNF . Nature 380, 252–255 (1996).
El-Agnaf, O. M. et al. Aggregates from mutant and wild-type α-synuclein proteins and NAC peptide induce apoptotic cell death in human neuroblastoma cells by formation of β-sheet and amyloid-like filaments. FEBS Lett. 440, 71–75 ( 1998).
Brandt, J et al. Trinucleotide repeat length and clinical progression in Huntington's disease. Neurology 46, 527– 531 (1996).
Sawa, A. et al. Increased apoptosis of Huntington disease lymphoblasts associated with repeat length-dependent mitochondrial depolarization. Nature Med. 5, 1194–1198 ( 1999).
Sanchez, I. et al. Caspase-8 is required for cell death induced by expanded polyglutamine repeats. Neuron 22, 623– 633 (1999).
Wellington, C. L. et al. Inhibiting caspase cleavage of huntingtin reduces toxicity and aggregate formation in neuronal and non-neuronal cells. J. Biol. Chem. 275, 19831–19838 (2000).
Leavitt, B. R., Wellington, C. I. & Hayden, M. R. Recent insights into the molecular pathogenesis of Huntington disease. Semin. Neurol. 19, 385 –395 (1999).
Ona, V. O. et al. Inhibition of caspase-1 slows disease progression in a mouse model of Huntington's disease. Nature 399, 263–267 (1999).
Senut, M. C., Suhr, S. T., Kaspar, B. & Gage, F. H. Intraneuronal aggregate formation and cell death after viral expression of expanded polyglutamine tracts in the adult rat brain. J. Neurosci. 20, 219–229 (2000).
Kim, M. et al. Mutant huntingtin expression in clonal striatal cells: dissociation of inclusion formation and neuronal survival by caspase inhibition. J. Neurosci. 19, 964–973 (1999).Provides insight into the pathogenic action of polyglutamine-repeat huntingtin in neuronal apoptosis.
Rigamonti, D. et al. Wild-type huntingtin protects from apoptosis upstream of caspase-3 . J. Neurosci. 20, 3705– 3713 (2000).
Cookson, M. R. & Shaw, P. J. Oxidative stress and motor neurone disease. Brain Pathol. 9, 165–186 (1999).
Smith, R. G. et al. Autoimmunity and ALS. Neurology 47, S40–S45 (1996).
Gurney, M. E. et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264, 1772–1775 (1994).
Wong, P. C. et al. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria . Neuron 14, 1105–1116 (1995).
Kruman, I., Pedersen, W. A. & Mattson, M. P. ALS-linked Cu/Zn-SOD mutation increases vulnerability of motor neurons to excitotoxicity by a mechanism involving increased oxidative stress and perturbed calcium homeostasis. Exp. Neurol. 160, 28–39 (1999).
Martin, L. J., Price, A. C., Kaiser, A., Shaikh, A. Y. & Liu, Z. Mechanisms for neuronal degeneration in amyotrophic lateral sclerosis and in models of motor neuron death. Int. J. Mol. Med. 5, 3–13 ( 2000).
Li, M. et al. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science 288, 335– 339 (2000).
Kostic, V., Jackson-Lewis, V., de Bilbao, F., Dubois-Dauphin, M. & Przedborski, S. Bcl-2: prolonging life in a transgenic mouse model of familial amyotrophic lateral sclerosis . Science 277, 559–562 (1997).
Dirnagl, U., Iadecola, C. & Moskowitz, M. A. Pathobiology of ischaemic stroke: an integrated view . Trends Neurosci. 22, 391– 397 (1999).Details molecular and cellular mechanisms in the neuronal death in ischaemic stroke.
Lipton, P. Ischemic cell death in brain neurons. Physiol. Rev. 79, 1431–568 (1999).
Yu, Z. et al. Pivotal role for acidic sphingomyelinase in cerebral ischemia-induced ceramide and cytokine production, and neuronal death. J. Mol. Neurosci. (in the press).
Bonventre, J. V. et al. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature 390, 622–625 (1997).
Schielke, G. P., Yang, G. Y., Shivers, B. D. & Betz, A. L. Reduced ischemic brain injury in interleukin-1β converting enzyme-deficient mice. J. Cereb. Blood Flow Metab. 18, 180 –185 (1998).
Hara, H. et al. Inhibition of interleukin 1 converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc. Natl Acad. Sci. USA 94, 2007–2012 ( 1997).
Murakami, K. et al. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J. Neurosci. 18, 205–213 ( 1998).
Keller, J. N. et al. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J. Neurosci. 18, 687–697 (1998).
Clark, R. S. et al. Increases in Bcl-2 and cleavage of caspase-1 and caspase-3 in human brain after head injury. FASEB J. 13, 813–821 (1999).
Fox, G. B., Fan, L., Levasseur, R. A. & Faden, A. I. Sustained sensory/motor and cognitive deficits with neuronal apoptosis following controlled cortical impact brain injury in the mouse. J. Neurotrauma 15, 599–614 ( 1998).
Napieralski, J. A., Raghupathi, R. & McIntosh, T. K. The tumor-suppressor gene, p53, is induced in injured brain regions following experimental traumatic brain injury. Mol. Brain Res. 71, 78–86 (1999).
Beer, R. et al. Expression of Fas and Fas ligand after experimental traumatic brain injury in the rat. J. Cereb. Blood Flow Metab. 20, 669–677 (2000).
Yakovlev, A. G. et al. Activation of CPP32-like caspases contributes to neuronal apoptosis and neurological dysfunction after traumatic brain injury. J. Neurosci. 17, 7415–7424 (1997).
Fink, K. B. et al. Reduction of post-traumatic brain injury and free radical production by inhibition of the caspase-1 cascade. Neuroscience 94, 1213–1218 ( 1999).
Sinson, G., Perri, B. R., Trojanowski, J. Q., Flamm, E. S. & McIntosh, T. K. Improvement of cognitive deficits and decreased cholinergic neuronal cell loss and apoptotic cell death following neurotrophin infusion after experimental traumatic brain injury. J. Neurosurg. 86, 511–518 (1997).
Albensi, B. C., Sullivan, P. G., Thompson, M. B., Scheff, S. W. & Mattson, M. P. Cyclosporine ameliorates traumatic brain injury-induced alterations of hippocampal synaptic plasticity. Exp. Neurol. 162, 385–389 (2000).
Emery, E. et al. Apoptosis after traumatic human spinal cord injury. J. Neurosurg. 89, 911–920 (1998).
Wada, S. et al. Apoptosis following spinal cord injury in rats and preventative effect of N-methyl-d-aspartate receptor antagonist. J. Neurosurg. 91, 98–104 ( 1999).
Springer, J. E., Azbill, R. D. & Knapp, P. E. Activation of the caspase-3 apoptotic cascade in traumatic spinal cord injury. Nature Med. 5, 943–946 (1999).
Crowe, M. J., Bresnahan, J. C., Shuman, S. L., Masters, J. N. & Beattie, M. S. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nature Med. 3, 73–76 (1997).
Ay, H., Ay, I., Koroshetz, W. J. & Finklestein, S. P. Potential usefulness of basic fibroblast growth factor as a treatment for stroke. Cerebrovasc. Dis. 9, 131–135 (1999).
The BDNF Study Group. A controlled trial of recombinant methionyl human BDNF in ALS: The BDNF Study Group (Phase III). Neurology 52, 1427–1433 ( 1999).
Borasio, G. D. et al. A placebo-controlled trial of insulin-like growth factor-I in amyotrophic lateral sclerosis. European ALS/IGF-I Study Group. Neurology 51, 583–586 ( 1998).
Grundman, M. Vitamin E and Alzheimer disease: the basis for additional clinical trials . Am. J. Clin. Nutr. 71, 630S– 636S (2000).
Logroscino, G. et al. Dietary lipids and antioxidants in Parkinson's disease: a population-based, case-control study. Ann. Neurol. 39, 89–94 (1996).
Duan, W. & Mattson, M. P. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson's disease. J. Neurosci. Res. 57, 195–206 (1999).
Ohlsson, A. L. & Johansson, B. B. Environment influences functional outcome of cerebral infarction in rats. Stroke 26, 644–649 ( 1995).
Mattson, M. P. & Duan, W. Apoptotic biochemical cascades in synaptic compartments: roles in adaptive plasticity and neurodegenerative disorders. J. Neurosci. Res. 58, 152– 166 (1999).Reviews the evidence for and implications of apoptosis-related mechanisms in synaptic remodelling and neuronal cell death.
Ivins, K. J., Bui, E. T. & Cotman, C. W. Beta-amyloid induces local neurite degeneration in cultured hippocampal neurons: evidence for neuritic apoptosis. Neurobiol. Dis. 5, 365–378 (1998).
Albensi, B. C. & Mattson, M. P. Evidence for the nvolvement TNF and NF-κB in hippocampal synaptic plasticity. Synapse 35, 151–159 ( 2000).
Author information
Authors and Affiliations
Glossary
- SYNAPTOGENESIS
-
The process of formation of synapses, the sites where neurons communicate through release of neurotransmitters from the presynaptic terminal and activation of receptors on the postsynaptic neuron.
- NEUROTROPHIC FACTORS
-
Proteins produced by neurons and glial cells that promote neuron survival and growth.
- METABOLIC STRESS
-
Conditions in which levels of glucose, oxygen and other molecules required for ATP (energy) production are decreased.
- LEUCINE ZIPPER
-
A leucine-rich domain within a protein that binds to other proteins with a similar domain.
- MICROGLIA
-
Phagocytic immune cells in the brain that engulf and remove cells that have undergone apoptosis.
- EXCITOTOXINS
-
Compounds such as glutamate, kainic acid and N-methyl-d-aspartate that can kill neurons by activating excitatory amino-acid (glutamate) receptors.
- LIMBIC STRUCTURES
-
Brain structures such as the hippocampus, amygdala and septum that function in learning and memory, and in emotions.
- NEURITES
-
Generic name for processes (axons and dendrites) elaborated from neuronal cell bodies.
- SYNAPTOSOME
-
A structure consisting of pre- and postsynaptic terminals prepared from homogenized brain tissue with cellular fractionation techniques.
- SUBSTANTIA NIGRA
-
A part of the midbrain that contains dopamine-producing neurons whose axons innervate the striatum and thereby control body movements.
- MITOCHONDRIAL COMPLEX I
-
A group of proteins located at the inner mitochondrial membrane that function very early in the electron transport chain.
- LEWY BODIES
-
Eosinophilic, cytoplasmic neuronal inclusions that contain aggregates of the proteins α-synuclein and ubiquitin.
- LYMPHOBLASTS
-
Bone-marrow-derived cells that give rise to lymphocytes.
- INTRANEURONAL INCLUSIONS
-
Aggregates of proteins that accumulate in neurons within the cytoplasm or nucleus.
- INFARCT
-
Brain tissue surrounding the site of a stroke in which cells die.
- ISCHAEMIC PENUMBRA
-
A region of tissue surrounding the necrotic core of an ischaemic infarct in which neurons die primarily by apoptosis.
- OLIGODENDROCYTES
-
A specific type of glial cell, which forms myelin membranes that insulate axons of neurons and thereby increase impulse conduction velocity.
Rights and permissions
About this article
Cite this article
Mattson, M. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol 1, 120–130 (2000). https://doi.org/10.1038/35040009
Issue Date:
DOI: https://doi.org/10.1038/35040009