Abstract
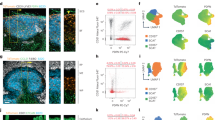
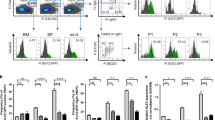
Lymphoid follicles are B-cell-rich compartments of lymphoid organs that function as sites of B-cell antigen encounter and differentiation. CXC chemokine receptor-5 (CXCR5) is required for B-cell migration to splenic follicles1, but the requirements for homing to B-cell areas in lymph nodes remain to be defined. Here we show that lymph nodes contain two types of B-cell-rich compartment: follicles containing follicular dendritic cells, and areas lacking such cells. Using gene-targeted mice, we establish that B-lymphocyte chemoattractant (BLC/BCA1)2,3 and its receptor, CXCR5, are needed for B-cell homing to follicles in lymph nodes as well as in spleen. We also find that BLC is required for the development of most lymph nodes and Peyer's patches. In addition to mediating chemoattraction, BLC induces B cells to upregulate membrane lymphotoxin α1β2, a cytokine that promotes follicular dendritic cell development and BLC expression4,5, establishing a positive feedback loop that is likely to be important in follicle development and homeostasis. In germinal centres the feedback loop is overridden, with B-cell lymphotoxin α1β2 expression being induced by a mechanism independent of BLC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Förster, R. et al. A putative chemokine receptor, BLR1, directs B-cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell 87, 1037–1047 (1996).
Gunn, M. D. et al. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature 391, 799–803 (1998).
Legler, D. F. et al. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J. Exp. Med. 187, 655–660 (1998).
Fu, Y. -X. & Chaplin, D. D. Development and maturation of secondary lymphoid tissues. Annu. Rev. Immunol. 17, 399–433 (1999).
Ngo, V. N. et al. Lymphotoxin-α/β and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J. Exp. Med. 189, 403– 412 (1999).
Tew, J. G. et al. Follicular dendritic cells and presentation of antigen and costimulatory signals to B cells. Immunol. Rev. 156, 39 –52 (1997).
Fu, Y. X., Huang, G., Wang, Y. & Chaplin, D. D. B lymphocytes induce the formation of follicular dendritic cell clusters in a lymphotoxin alpha-dependent fashion. J. Exp. Med. 187, 1009–1018 (1998).
Gonzalez, M., Mackay, F., Browning, J. L., Kosco-Vilbois, M. H. & Noelle, R. J. The sequential role of lymphotoxin and B cells in the development of splenic follicles. J. Exp. Med. 187, 997–1007 (1998).
Endres, R. et al. Mature follicular dendritic cell networks depend on expression of lymphotoxin beta receptor by radioresistant stromal cells and of lymphotoxin beta and tumor necrosis factor by B cells. J. Exp. Med. 189, 159–168 (1999).
Browning, J. L. et al. Lymphotoxin beta, a novel member of the TNF family that forms a heteromeric complex with lymphotoxin on the cell surface. Cell 72, 847–856 ( 1993).
Worm, M. & Geha, R. S. CD40 ligation induces lymphotoxin alpha gene expression in human B cells. Int. Immunol. 6, 1883–1890 (1994).
Pokholok, D. K. et al. Cloning and expression analysis of the murine lymphotoxin beta gene. Proc. Natl Acad. Sci. USA 92, 674–678 (1995).
Browning, J. L. et al. Characterization of lymphotoxin-alpha beta complexes on the surface of mouse lymphocytes. J. Immunol. 159, 3288–3298 (1997).
Miller, J. J. D., Johnsen, D. O. & Ada, G. L. Differences in localization of Salmonella flagella in lymph node follicles of germ-free and conventional rats. Nature 217, 1059–1061 ( 1968).
Mason, D. Y., Jones, M. & Goodnow, C. C. Development and follicular localization of tolerant B lymphocytes in lysozyme/anti-lysozyme IgM/IgD transgenic mice. Int. Immunol. 4, 163–175 (1992).
Gutman, G. A. & Weissman, I. L. Homing properties of thymus-independent follicular lymphocytes. Transplantation 16, 621–629 (1973).
Kawabe, T. et al. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity 1, 167–178 (1994).
Vajdy, M., Kosco-Vilbois, M. H., Kopf, M., Kohler, G. & Lycke, N. Impaired mucosal immune responses in interleukin 4-targeted mice. J. Exp. Med. 181, 41–53 (1995).
Cyster, J. G. Chemokines and cell migration in secondary lymphoid organs. Science 286, 2098–2102 ( 1999).
Voigt, I. et al. CXCR5-deficient mice develop functional germinal centers in the splenic T cell zone. Eur. J. Immunol. 30, 560–567 (2000).
Mackay, F. & Browning, J. L. Turning off follicular dendritic cells. Nature 395, 26–27 (1998).
Golovkina, T. V., Shlomchik, M., Hannum, L. & Chervonsky, A. Organogenic role of B lymphocytes in mucosal immunity. Science 286, 1965–1968 ( 1999).
Luther, S. A., Lopez, T., Bai, W., Hanahan, D. & Cyster, J. G. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity 12, 471–481 ( 2000).
Yokota, Y. et al. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature 397, 702–706 (1999).
Mebius, R. E., Rennert, P. & Weissman, I. L. Developing lymph nodes collect CD4+CD3-LTβ+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 7, 493–504 (1997).
Yoshida, H. et al. IL-7 receptor α+ CD3- cells in the embryonic intestine induces the organizing center of Peyer's patches. Int. Immunol. 11, 643– 655 (1999).
Matsumoto, M. et al. Role of lymphotoxin and the type I TNF receptor in the formation of germinal centers. Science 271, 1289– 1291 (1996).
Korner, H. et al. Distinct roles for lymphotoxin-alpha and tumor necrosis factor in organogenesis and spatial organization of lymphoid tissue. Eur. J. Immunol. 27, 2600–2609 (1997).
Ansel, K. M., McHeyzer-Williams, L. J., Ngo, V. N., McHeyzer-Williams, M. G. & Cyster, J. G. In vivo activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J. Exp. Med. 190, 1123–1134 (1999).
Tilney, N. L. Patterns of lymphatic drainage in the adult laboratory rat. J. Anat. 109, 369–383 ( 1971).
Acknowledgements
We are especially grateful to N. Killeen for advice and help in generating the BLC knockout mice; K. Reif and L. Tang for helpful advice; and A. Schmidt and C. Backus for blastocyst transfers. K.M.A. is a HHMI predoctoral fellow, S.A.L. is supported by Human Frontier Science Program, and J.G.C is a Packard fellow. This work was supported by grants from the NIH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ansel, K., Ngo, V., Hyman, P. et al. A chemokine-driven positive feedback loop organizes lymphoid follicles . Nature 406, 309–314 (2000). https://doi.org/10.1038/35018581
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35018581
This article is cited by
-
B cell development and antibody responses in human immune system mice: current status and future perspective
Science China Life Sciences (2024)
-
CXCL13 contributes to chronic pain of a mouse model of CRPS-I via CXCR5-mediated NF-κB activation and pro-inflammatory cytokine production in spinal cord dorsal horn
Journal of Neuroinflammation (2023)
-
Immune subset-committed proliferating cells populate the human foetal intestine throughout the second trimester of gestation
Nature Communications (2023)
-
Long-term retention of antigens in germinal centers is controlled by the spatial organization of the follicular dendritic cell network
Nature Immunology (2023)
-
γδ T cells: origin and fate, subsets, diseases and immunotherapy
Signal Transduction and Targeted Therapy (2023)