Abstract
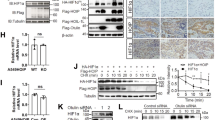
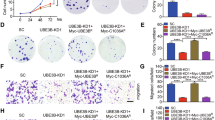
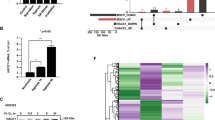
von Hippel–Lindau (VHL) disease is a hereditary cancer syndrome that is characterized by the development of multiple vascular tumors and is caused by inactivation of the von Hippel–Lindau protein (pVHL). Here we show that pVHL, through its β-domain, binds directly to hypoxia-inducible factor (HIF), thereby targeting HIF for ubiquitination in an α-domain-dependent manner. This is the first function to be ascribed to the pVHL β-domain. Furthermore, we provide the first direct evidence that pVHL has a function analogous to that of an F-box protein, namely, to recruit substrates to a ubiquitination machine. These results strengthen the link between overaccumulation of HIF and development of VHL disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Stebbins, C. E., Kaelin, W. G. J. & Pavletich, N. P. Structure of the VHL–elonginC–elonginB complex: implications for VHL tumor suppressor function. Science 284, 455–461 ( 1999).
Kaelin, W. G. Cancer. Many vessels, faulty gene. Nature 399, 203–204 (1999).
Lisztwan, J., Imbert, G., Wirbelauer, C., Gstaiger, M. & Krek, W. The von Hippel–Lindau tumor suppressor protein is a component of an E3 ubiquitin-protein ligase activity. Genes Dev. 13, 1822–1833 (1999).
Iwai, K. et al. Identification of the von Hippel–Lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex. Proc. Natl Acad. Sci. USA 96, 12436–12441 (1999).
Maxwell, P. et al. The von Hippel–Lindau gene product is necessary for oxgyen-dependent proteolysis of hypoxia-inducible factor α subunits . Nature 399, 271–275 (1999).
Huang, L. E., Gu, J., Schau, M. & Bunn, H. F. Regulation of hypoxia-inducible factor 1 alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc. Natl Acad. Sci. USA 95, 7987–7992 ( 1998).
Kallio, P., Wilson, W., O’Brien, S., Makino, Y. & Poellinger, L. Regulation of the hypoxia-inducible transcription factor 1 alpha by the ubiquitin-proteasome pathway. J. Biol. Chem. 274, 6519–6525 (1999).
Pugh, C., O’Rourke, J., Nagao, M., Gleadle, J. & Ratcliffe, P. Activation of hypoxia-inducible factor-1: definition of regulatory domains within the alpha subunit. J. Biol. Chem. 272, 11205–11214 (1997).
Ohh, M. & Kaelin, W. G. J. The von Hippel–Lindau tumour suppressor protein: new perspectives. Mol. Med. Today 5, 257–263 (1999).
Kamura, T. et al. The Elongin BC complex interacts with the conserved SOCS-box motif present in members of the SOCS, ras, WD-40 repeat, and ankyrin repeat families. Genes Dev. 12, 3872– 3881 (1998).
Duan, D. R. et al. Inhibition of transcriptional elongation by the VHL tumor suppressor protein. Science 269, 1402– 1406 (1995).
Kibel, A., Iliopoulos, O., DeCaprio, J. D. & Kaelin, W. G. Binding of the von Hippel–Lindau tumor suppressor protein to elongin B and C. Science 269, 1444– 1446 (1995).
Salceda, S. & Caro, J. Hypoxia-inducible factor 1 alpha (HIF-1 alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J. Biol. Chem. 272, 22642– 2267 (1997).
Ohh, M. et al. Synthetic peptides define critical contacts between elongin C, elongin B, and the von Hippel–Lindau protein. J. Clin. Invest. 104, 1583–1591 (1999).
An, W. et al. Stabilization of wild-type p53 by hypoxia-inducible factor 1 alpha . Nature 392, 405–408 (1998).
Chandel, N. et al. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl Acad. Sci. USA 95, 11715–11720 (1998).
Ritter, M. et al. Isolated familial pheochromocytoma as a variant of von Hippel–Lindau Disease. J. Clin. Endocrinol. Met. 81, 1035 –1037 (1996).
Crossey, P. A. et al. Identification of intragenic mutations in the von Hippel–Lindau disease tumor suppressor gene and correlation with disease phenotype. Hum. Mol. Genet. 3, 1303–1308 (1994).
Iliopoulos, O., Kibel, A., Gray, S. & Kaelin, W. G. Tumor suppression by the human von Hippel–Lindau gene product. Nature Med. 1, 822–826 ( 1995).
Lonergan, K. M. et al. Regulation of hypoxia-inducible mRNAs by the von Hippel–Lindau protein requires binding to complexes containing elongins B/C and Cul2. Mol. Cell Biol. 18, 732–741 (1998).
Acknowledgements
We thank C. Stebbins for purified pVHL/elongin C/elongin B complexes and H. Franklin Bunn and M. Meyerson for critical reading of the manuscript. This work was supported by grants from the NIH and from the Murray Foundation. M.O. is a Fellow of the National Cancer Institute of Canada. W.G.K. is a Howard Hughes Medical Institute assistant investigator.
Correspondence and requests for materials should be addressed to W.G.K.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ohh, M., Park, C., Ivan, M. et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the β-domain of the von Hippel–Lindau protein. Nat Cell Biol 2, 423–427 (2000). https://doi.org/10.1038/35017054
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35017054
This article is cited by
-
Glycolytic enzymes in non-glycolytic web: functional analysis of the key players
Cell Biochemistry and Biophysics (2024)
-
Potential contribution of PEP carboxykinase-dependent malate dismutation to the hypoxia response in C. elegans
Nature Communications (2023)
-
A guide to cell death pathways
Nature Reviews Molecular Cell Biology (2023)
-
GPER-mediated stabilization of HIF-1α contributes to upregulated aerobic glycolysis in tamoxifen-resistant cells
Oncogene (2023)
-
Belzutifan in adults with VHL-associated central nervous system hemangioblastoma: a single-center experience
Journal of Neuro-Oncology (2023)