Abstract
Pathogenic bacteria are becoming increasingly resistant to common antibiotics, stimulating an intensive search for new ones. Knowing that a class of medium-sized peptides (magainins1) are widely used by host organisms as a defence against microbial invasion2, we developed a β-amino-acid oligomer (β-peptide) that mimics these natural antibiotics and tested it for antimicrobial activity. We find not only that the activity of our β-peptide is comparable to that of a magainin derivative but also that it is effective against four bacterial species, including two pathogens that are resistant to common antibiotics.
Similar content being viewed by others
Main
Natural peptide antibiotics are highly diverse in terms of size, sequence and conformation2; they are cationic and often adopt amphiphilic secondary structures. One common class, exemplified by the magainins1, features 20- to 30-residue peptides that form amphiphilic α-helices (hydrophobic side chains on one side of the helix and cationic side chains on the other) which are attracted to the negatively charged surfaces of bacteria. These helices then somehow disrupt the bacterial membrane3,4,5.
β-peptides are promising antimicrobial candidates because they offer a choice of secondary structures6,7,8 and because the unnatural β-peptide backbone is resistant to protease degradation9, in contrast to the α-amino-acid backbone of conventional peptides. Three distinct helical conformations have been identified among β-peptides, with the helix type being determined by the substitution pattern on the β-amino-acid residues6,7,8. Oligomers of (R,R)-trans-2-aminocyclopentanecarboxylic acid (ACPC) adopt a helix defined by a 12-membered ring formed as a result of hydrogen-bonding between each backbone carbonyl group and the amide proton of the third residue in the carboxy-terminal direction (a 12-helix)10.
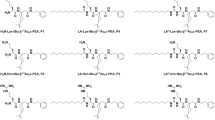
To test our amphiphilic versions of the β-peptide 12-helix for antimicrobial activity, we used (3R,4S)-trans-4-aminopyrrolidine-3-carboxylic acid (APC), together with ACPC, to prepare a β-17 oligomer ( Fig. 1a). The APC residue should be cationic at pH 8 or below, by virtue of ring-nitrogen protonation. Because the 12-helix has about 2.5 residues per turn, the 12-helical conformation of β-17 should be amphiphilic, with all hydrophilic APC residues on one side of the helix and all hydrophobic ACPC residues on the other (Fig. 1b).
a, Chemical structure of β-17. b, Axial projection of the β-peptide 12-helix, highlighting the ∼5-residue repeat that results from there being approximately 2.5 residues per 12-helical turn. The repeating pentad in β-17, +H+HH (+, cationic APC residue; H, hydrophobic ACPC residue), is shown. c, Haemolytic activity of β-17 (squares), the magainin derivative (crosses), and melittin (circles). Human red blood cells (hRBC, 1% suspension in PBS buffer) were incubated at room temperature for 1 h with a twofold serial dilution of peptide in PBS buffer. Release of haemoglobin was determined from the absorbance at 415 nm of the supernatant after centrifugation. Controls, hRBC suspended in PBS (0% hydrolysis) or in 1% SDS (100% haemolysis).
We compared the activities of β-17 and the synthetic magainin derivative11 GIGKFLHAAKKFAKAFVAEIMNS-NH2 against four bacteria (Table 1). The bacterium Enterococcus faecium A436 (which is vancomycin resistant) and Staphylococcus aureus 5332 (methicillin resistant) are clinical isolates, whereas Bacillus subtilis BR151 and Escherichia coli JM109 are non-pathogenic strains commonly used in the laboratory for genetic construction. We find that the activity of β-17 is comparable to that of the magainin against all four species of bacteria.
To be useful therapeutically, this antimicrobial action must be effective in the presence of human cells. We therefore tested the effect of our β-peptide on red blood cells, knowing that although magainins themselves are only weakly haemolytic (cause red blood cells to break open), other natural cationic helix-forming peptides, such as melittin12, are strongly haemolytic. Figure 1c compares haemolysis by melittin (a positive control), the magainin derivative, and β-17: the β-peptide has even less haemolytic activity than the magainin. Another class of β-peptides composed of acyclic residues are also potent against E. coli, but these are highly haemolytic13, limiting their therapeutic application.
We have devised a hetero-oligomer with an unnatural backbone that can be used to mimic a specific and useful biological activity displayed by a naturally defensive, medium-sized peptide. The chemical and conformational stability of β-peptides may lead to the creation of a new class of anti-microbial agents, which will add to their other unusual clinical applications14.
References
Zasloff, M. Proc. Natl Acad. Sci. USA 84, 5449– 5453 (1987).
Boman, H. G. Annu. Rev. Immunol. 13, 61–92 (1995).
Saberwal, G. & Nagaraj, R. Biochim. Biophys. Acta 1197, 109–131 (1994).
Oren, Z. & Shai, Y. Biopolymers 47, 451–463 (1999).
Wu, M., Maier, E., Benz, R. & Hancock, R. E. W. Biochemistry 38, 7235–7242 ( 1999).
Seebach, D. & Matthews, J. L. J. Chem. Soc. Chem. Commun. 2015–2022 (1997).
Gellman, S. H. Acc. Chem. Res. 31, 173–180 (1998).
DeGrado, W. F., Schneider, J. P. & Hamuro, Y. J. Peptide Res. 54, 206– 217 (1999).
Hintermann, T. & Seebach, D. Chimia 51, 244–248 (1997).
Appella, D. H. et al. Nature 387, 381–384 (1997).
Chen, H.-C, Brown, J. H., Morell, J. L. & Huang, C. M. FEBS Lett. 236, 462–466 (1988).
Habermann, E. Science 177, 314–322 ( 1972).
Hamuro, Y., Schneider, J. P. & DeGrado, W. F. J. Am. Chem. Soc. 121, 12200 –12201 (1999).
Werder, M., Hauser, H., Abele, S. & Seebach, D. Helv. Chim. Acta 82, 1774–1783 ( 1999).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Porter, E., Wang, X., Lee, HS. et al. Non-haemolytic β-amino-acid oligomers . Nature 404, 565 (2000). https://doi.org/10.1038/35007145
Issue Date:
DOI: https://doi.org/10.1038/35007145