Abstract
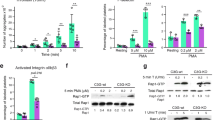
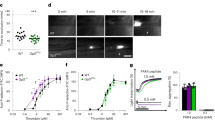
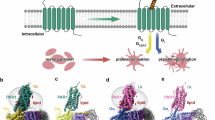
Identification of the mechanisms by which the coagulation protease thrombin activates platelets is critical for understanding haemostasis and thrombosis. Thrombin activates cells at least in part by cleaving protease-activated G-protein-coupled receptors (PARs)1. PAR3 and PAR4 are thrombin receptors expressed in mouse platelets2,3. Inhibition of thrombin binding to mPAR3 (ref. 4) and knockout of the mPAR3 gene3 inhibited mouse platelet activation at low but not high concentrations of thrombin. Thus PAR3 is important for thrombin signalling in mouse platelets. Expression of human PAR3 in heterologous expression systems reliably resulted in responsiveness to thrombin2. Curiously, despite its importance for the activation of mouse platelets by thrombin3,4, mouse PAR3 (mPAR3) did not lead to thrombin signalling even when overexpressed. We now report that mPAR3 and mPAR4 interact in a novel way: mPAR3 does not itself mediate transmembrane signalling but instead functions as a cofactor for the cleavage and activation of mPAR4 by thrombin. This establishes a paradigm for cofactor-assisted PAR activation and for a G-protein-coupled receptor's acting as an accessory molecule to present ligand to another receptor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Coughlin,S. R. How the protease thrombin talks to cells. Proc. Natl Acad. Sci. USA 96, 11023–11027 ( 1999).
Ishihara,H. et al. Protease-activated receptor 3 is a second thrombin receptor in humans. Nature 386, 502– 506 (1997).
Kahn,M. L. et al. A dual thrombin receptor system for platelet activation. Nature 394, 690–694 ( 1998).
Ishihara,H., Zeng,D., Connolly,A. J., Tam,C. & Coughlin,S. R. Antibodies to protease-activated receptor 3 inhibit activation of mouse platelets by thrombin. Blood 91, 4152–4157 (1998).
Kuner,R. et al. Role of heteromer formation in GABAB receptor function. Science 283, 74–77 ( 1999).
Jordan,B. A. & Devi,L. A. G-protein-coupled receptor heterodimerization modulates receptor function. Nature 399, 697–699 (1999).
White,J. H. et al. Heterodimerization is required for the formation of a functional GABAB receptor. Nature 396, 679 –682 (1998).
Kaupmann,K. et al. GABAB-receptor subtypes assemble into functional heteromeric complexes. Nature 396, 683– 687 (1998).
Kahn,M. L., Nakanishi-Matsui,M., Shapiro, M. J., Ishihara,H. & Coughlin,S. R. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J. Clin. Invest. 103, 879–887 ( 1999).
Vu,T. -K. H., Wheaton,V. I., Hung,D. T. & Coughlin,S. R. Domains specifying thrombin–receptor interaction. Nature 353, 674–677 ( 1991).
Liu,L., Vu,T. -K. H., Esmon,C. T. & Coughlin,S. R. The region of the thrombin receptor resembling hirudin binds to thrombin and alters enzyme specificity. J. Biol. Chem. 266, 16977–16980 (1991).
Mathews,I. I. et al. Crystallographic structures of thrombin complexed with thrombin receptor peptides: existence of expected and novel binding modes. Biochemistry 33, 3266–3279 (1994).
Ishii,K., Gerszten,R., Zheng,Y. -W., Turck,C. W. & Coughlin,S. R. Determinants of thrombin receptor cleavage: receptor domains involved, specificity, and role of the P3 aspartate. J. Biol. Chem. 270, 16435– 16440 (1995).
Ishii,K., Hein,L., Kobilka,B. & Coughlin,S. R. Kinetics of thrombin receptor cleavage on intact cells. Relation to signaling. J. Biol. Chem. 268, 9780–9786 (1993).
Vu,T. -K. H., Hung,D. T., Wheaton,V. I. & Coughlin,S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 64, 1057–1068 (1991).
Xu,W. F. et al. Cloning and characterization of human protease-activated receptor 4. Proc. Natl Acad. Sci. USA 95, 6642– 6646 (1998).
Okamura,T., Hasitz,M. & Jamieson, G. A. Platelet glycocalicin: interaction with thrombin and role as thrombin receptor on the platelet surface. J. Biol. Chem. 253, 3435–3443 ( 1978).
Colman,R. W., Marder,V. J., Salzman,E. W. & Hirsh,J. in Hemostasis and Thrombosis (eds Colman, R. W., Marder, V. J., Salzman, E. W. & Hirsh, J.) 3–18 (Lippincott, Philadelphia, 1994).
Ulevitch,R. J. & Tobias,P. S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu. Rev. Immunol. 13, 437–457 ( 1995).
Chow,J. C., Young,D. W., Golenbock,D. T., Christ,W. J. & Gusovsky,F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274, 10689–10692 (1999).
Heinrich,P. C., Behrmann,I., Müller-Newen, G., Schaper,F. & Graeve,L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem. J. 334 , 297–314 (1998).
Klagsbrun,M. Mediators of angiogenesis: the biological significance of basic fibroblast growth factor (bFGF)–heparin and heparan sulfate interactions. Semin. Cancer Biol. 3, 81–87 (1992).
Vivien,D., Attisano,L., Wrana,J. L. & Massagué,J. Signaling activity of homologous and heterologous transforming growth factor-beta receptor kinase complexes. J. Biol. Chem. 270, 7134 –7141 (1995).
Chen,J., Ishii,M., Wang,L., Ishii,K. & Coughlin,S. R. Thrombin receptor activation: confirmation of the intramolecular tethered liganding hypothesis and discovery of an alternative intermolecular liganding mode. J. Biol. Chem. 269, 16041 –16045 (1994).
Sage,S. O. in Platelets, A Practical Approach (eds Watson, S. P. & Authi, K. S.) 67–90 (IRL, Oxford, 1996).
Acknowledgements
We thank H. Bourne, T. Nakanishi, M. Shapiro and J. Trejo for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakanishi-Matsui, M., Zheng, YW., Sulciner, D. et al. PAR3 is a cofactor for PAR4 activation by thrombin. Nature 404, 609–613 (2000). https://doi.org/10.1038/35007085
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35007085