Abstract
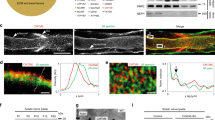
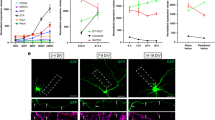
Adult mammalian axon regeneration is generally successful in the peripheral nervous system (PNS) but is dismally poor in the central nervous system (CNS). However, many classes of CNS axons can extend for long distances in peripheral nerve grafts1. A comparison of myelin from the CNS and the PNS has revealed that CNS white matter is selectively inhibitory for axonal outgrowth2. Several components of CNS white matter, NI35, NI250(Nogo) and MAG, that have inhibitory activity for axon extension have been described3,4,5,6,7. The IN-1 antibody, which recognizes NI35 and NI250(Nogo), allows moderate degrees of axonal regeneration and functional recovery after spinal cord injury8,9. Here we identify Nogo as a member of the Reticulon family, Reticulon 4-A. Nogo is expressed by oligodendrocytes but not by Schwann cells, and associates primarily with the endoplasmic reticulum. A 66-residue lumenal/extracellular domain inhibits axonal extension and collapses dorsal root ganglion growth cones. In contrast to Nogo, Reticulon 1 and 3 are not expressed by oligodendrocytes, and the 66-residue lumenal/extracellular domains from Reticulon 1, 2 and 3 do not inhibit axonal regeneration. These data provide a molecular basis to assess the contribution of Nogo to the failure of axonal regeneration in the adult CNS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Benfy, M. & Aguayo, A. G. Extensive elongation of axons from rat brain into peripheral nerve grafts. Nature 296, 150–152 (1982).
Schwab, M. E. & Thoenen, H. Dissociated neurons regenerate into sciatic but not optic nerve explants in culture irrespective of neurotrophic factors. J. Neurosci. 5, 2415–2423 (1985).
Wang, L. H. et al. in Neurobiology of Spinal Cord Injury (eds Kalb, R. G. & Strittmatter, S. M.) 131–153 (Humana, Totowa, New Jersey, 1999).
Caroni, P. & Schwab, M. E. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J. Cell Biol. 106, 1281–1288 (1988).
Spillmann, A. A., Bandtlow, C. E., Lottspeich, F., Keller, F. & Schwab, M. E. Identification and characterization of a bovine neurite growth inhibitor (bNI-220). J. Biol. Chem. 73, 19283–19293 (1998).
McKerracher, L. et al. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron 13, 805–811 (1994).
Mukhopadhyay, G., Doherty, P., Walsh, F. S., Crocker, R. & Filbin, M. T. A novel role of myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron 13, 757–767 (1994).
Bregman, B. S. et al. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature 378, 498–501 (1995).
Thallmair, M. et al. Neurite growth inhibitors restrict plasticity and functional recovery following corticospinal tract lesions. Nature Neurosci. 1, 24–31 (1998).
Nagase, T. et al. Prediction of the coding sequences of unidentified human genes. XII. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 31, 355–364 (1998).
van de Velde, H. J., Roebroek, A. J., Senden, N. H., Ramaekers, F. C. & Van de Ven, W. J. NSP-encoded reticulons, neuroendocrine proteins of a novel gene family associated with membranes of the endoplasmic reticulum. J. Cell Sci. 107, 2403–2416 (1994).
Roebroek, A. J. et al. Genomic organization of the human NSP gene, prototype of a novel gene family encoding reticulons. Genomics 32, 191–199 (1996).
Roebroek, A. J., Contreras, B., Pauli, I. G. & Van de Ven, W. J. cDNA cloning, genomic organization, and expression of the human RTN2 gene, a member of a gene family encoding reticulons. Genomics 51, 98–106 (1998).
Moreira, E. F., Jaworski, C. J. & Rodriguez, I. R. Cloning of a novel member of the reticulon gene family (RTN3): gene structure and chromosomal localization to 11q13. Genomics 58, 73–81 (1999).
Morris, N. J., Ross, S. A., Neveu, J. M., Lane, W. S. & Lienhard, G. E. Cloning and characterization of a new member of the reticulon family. Biochim. Biophys. Acta 1450, 68–76 (1991).
Jackson, M. R., Nilsson, T. & Peterson, P. A. Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J. 9, 3153–3162 (1991).
Senden, N. H. et al. Neuroendocrine-specific protein C (NSP-C): subcellular localization and differential expression in relation to NSP-A. Eur. J. Cell Biol. 69, 197–213 (1996).
Bandtlow, C. E. & Loschinger, J. Developmental changes in neuronal responsiveness to the CNS myelin-associated neurite growth inhibitor NI-35/250. Eur. J. Neurosci. 9, 2743–2752 (1997).
Takahashi, T., Nakamura, F., Jin, Z., Kalb, R. G. & Strittmatter, S. M. Semaphorin A and E act as antagonists of neuropilin-1 and agonists of neuropilin-2 receptors. Nature Neurosci. 1, 487–493 (1998).
Takahashi, T. et al. Plexin-neuropilin1 complexes form functional semaphorin-3A receptors. Cell 99, 59–69 (1999).
Goshima, Y., Nakamura, F., Strittmatter, P. & Strittmatter, S. M. Collapsin-induced growth cone collapse mediated by an intracellular protein related to unc-33. Nature 376, 509–514 (1995).
Wang, L. H. & Strittmatter, S. M. A family of rat CRMP genes is differentially expressed in the nervous system. J. Neurosci. 16, 6197–6207 (1996).
Jin, Z. & Strittmatter, S. M. Rac1 mediates collapsin-induced growth cone collapse. J. Neurosci. 17, 6256–6263 (1997).
Strittmatter, S. M., Fishman, M. C. & Zhu, X. P. Activated mutants of the α subunit of Go promote an increased number of neurites per cell. J. Neurosci. 14, 2327–2338 (1994).
Vartanian, T., Fischbach, G. & Miller, R. Failure of spinal cord oligodendrocyte development in mice lacking neuregulin. Proc. Natl Acad. Sci. USA 96, 731–735 (1999).
Acknowledgements
The authors thank A. Fournier for invaluable experimental advice and assistance. This work was supported by grants to S.M.S. from the NIH and the American Paralysis Association, and to F.N. from the Spinal Cord Research Fund of the Paralyzed Veterans of America. S.M.S. is an Investigator of the Patrick and Catherine Weldon Donaghue Medical Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
GrandPré, T., Nakamura, F., Vartanian, T. et al. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature 403, 439–444 (2000). https://doi.org/10.1038/35000226
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35000226