Abstract
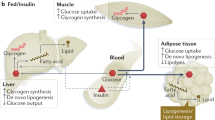
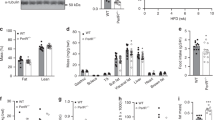
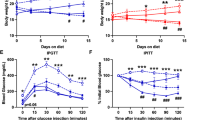
Type 2 diabetes is a complex metabolic disorder characterized by peripheral insulin resistance and impaired Β cell function1,2. Insulin resistance is inherited as a non-mendelian trait3. In genetically predisposed individuals, resistance of skeletal muscle and adipose tissue to insulin action precedes the onset of clinical diabetes, and is thought to contribute to hyperglycaemia by leading to impaired Β cell function and increased hepatic glucose production4,5. It is not clear whether Β cell and liver defects are also genetically determined2. To test the hypothesis that insulin resistance in muscle and fat is sufficient to cause type 2 diabetes in the absence of intrinsic Β cell and liver abnormality, we generated transgenic mice that were insulin-resistant in skeletal muscle and adipose tissue. These mice developed all the prodromal features of type 2 diabetes but, despite the compounded effect of peripheral insulin resistance and a mild impairment of Β cell function, failed to become diabetic. These findings indicate the need for a critical re-examination of the primary site(s) of insulin resistance in diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Bogardus, C. Metabolic abnormalities in the development of non-insulin-dependent diabetes mellitus. in Diabetes Mellitus: A Fundamental and Clinical Text (eds LeRoith, D., Taylor, S.I. & Olefsky, G.M.) 459– 467 (Lippincott-Raven, New York, 1996).
Polonsky, K.S., Sturis, J. & Bell, G.I. Non-insulin-dependent diabetes mellitus—a genetically programmed failure of the Β cell to compensate for insulin resistance. N. Engl. J. Med. 334, 777– 783 (1996).
Kahn, C.R., Vicent, D. & Doria, A. Genetics of non-insulin-dependent (type-II) diabetes mellitus. Annu. Rev. Med. 47, 509– 531 (1996).
DeFronzo, R.A. Lilly lecture 1987. The triumvirate: β-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 37, 667– 687 (1988).
Reaven, G.M. Pathophysiology of insulin resistance in human disease. Physiol. Rev. 75, 473–486 (1995).
Accili, D. et al. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nature Genet. 12, 106–109 (1996).
Bruning, J.C. et al. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell 88, 561–572 (1997).
Wang, L. et al. Hyperinsulinemia but no diabetes in transgenic mice homozygously expressing the tyrosine kinase-deficient human insulin receptor. Biochem. Biophys. Res. Commun. 240, 446– 451 (1997).
Nishiyama, T. et al. Expression of the gene encoding the tyrosine kinase-deficient human insulin receptor in transgenic mice. Gene 141, 187–192 (1994).
Ebina, Y. et al. Replacement of lysine residue 1030 in the putative ATP-binding region of the insulin receptor abolishes insulin- and antibody-stimulated glucose uptake and receptor kinase activity. Proc. Natl Acad. Sci. USA 84, 704–708 (1987).
Di Cola, G., Cool, M.H. & Accili, D. Hypoglycemic effect of insulin-like growth factor-1 in mice lacking insulin receptors. J. Clin. Invest. 99, 2538–2544 (1997).
Unger, R.H. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes 44, 863– 870 (1995).
Zhou, Y.P. & Grill, V.E. Long-term exposure of rat pancreatic islets to fatty acids inhibits glucose-induced insulin secretion and biosynthesis through a glucose fatty acid cycle. J. Clin. Invest. 93, 870–876 (1994).
Uysal, K.T., Wiesbrock, S.M., Marino, M.W. & Hotamisligil, G.S. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature 389, 610– 614 (1997).
Leibiger, I.B., Leibiger, B., Moede, T. & Berggren, P.O. Exocytosis of insulin promotes insulin gene transcription via the insulin receptor/PI-3 kinase/p70 s6 kinase and CaM kinase pathways. Mol. Cell 1, 933–938 (1998).
Withers, D.J. et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391, 900–904 (1998).
Araki, E. et al. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature 372, 186–190 (1994).
Tamemoto, H. et al. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature 372, 182–186 (1994).
Chang, P.Y. et al. Expression of a dominant-negative mutant human insulin receptor in the muscle of transgenic mice. J. Biol. Chem. 269, 16034–16040 (1994).
Weber, T.M., Joost, H.G., Simpson, I.A. & Cushman, S.W. in The Insulin Receptor (Alan R. Liss, New York, 1988).
Tolman, E.L., Schworer, C.M. & Jefferson, L.S. Effects of hypophysectomy on amino acid metabolism and gluconeogenesis in the perfused rat liver. J. Biol. Chem. 248, 4552–4560 (1973).
Cinti, S., Eberbach, S., Castellucci, M. & Accili, D. Lack of insulin receptors affects the formation of white adipose tissue in mice. A morphological and ultrastructural analysis. Diabetologia 41, 171–177 (1998).
Boschero, A.C. et al. Oxotremorine-m potentiation of glucose-induced insulin release from rat islets involves M3 muscarinic receptors. Am. J. Physiol. 268, E336–342 (1995).
Acknowledgements
We thank P. Rogliani for help with the Pck2 measurements, J. Nakae for the analysis of insulin receptor expression in islets, I. Atwater and A. Cotterell for performing islet microdissection and perfusion experiments. We also thank C.R. Kahn for helpful comments and sharing unpublished information, S. Cushman for support and advice and K. Rother for critical reading of the manuscript. The work was supported in part by a grant from the American Diabetes Association and by a generous gift of Sigma Tau Corp. to D.A. D.L. is supported by a fellowship grant from the University of Rome 'La Sapienza'.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lauro, D., Kido, Y., Castle, A. et al. Impaired glucose tolerance in mice with a targeted impairment of insulin action in muscle and adipose tissue. Nat Genet 20, 294–298 (1998). https://doi.org/10.1038/3112
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/3112