Abstract
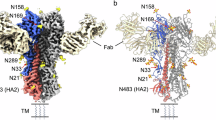
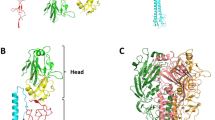
The haemagglutinin glycoprotein of influenza virus is a trimer comprising two structurally distinct regions: a triple-stranded coiled-coil of α-helices extends 76 Å from the membrane and a globular region of antiparallel β-sheet, which contains the receptor binding site and the variable antigenic determinants, is positioned on top of this stem. Each subunit has an unusual loop-like topology, starting at the membrane, extending 135 Å distally and folding back to enter the membrane.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Laver, W. G. & Kilbourne, E. D. Virology 30, 493–501 (1966).
Brand, C. M. & Skehel, J. J. Nature new Biol. 238, 145–147 (1972).
Wiley, D. C. & Skehel, J. J. Topics infect. Dis. 3, 135–138 (1978).
Skehel, J. J. & Waterfield, M. D. Proc. natn. Acad. Sci. U.S.A. 72, 93–97 (1975).
Porter, A. G. et al. Nature 282, 471–477 (1979).
Ward, C. W. & Dopheide, T. A. in Structure and Variation in Influenza Virus (eds Laver, W. G. & Air, G. M.) 27–38 (Elsevier, Amsterdam, 1980).
Dopheide, T. A. & Ward, C. W. in Structure and Variation in Influenza Virus (eds Laver, W. G. & Air, G. M.) 21–26 (Elsevier, Amsterdam, 1980).
Sleigh, M. J., Both, G. W., Brownlee, G. G., Bender, V. J. & Moss, B. A. in Structure and Variation in Influenza Virus (eds Laver, W. G. & Air, G. M.) 69–78 (Elsevier, Amsterdam, 1980).
Gething, M.J., Skehel, J. & Waterfield, M. D. in Structure and Variation in Influenza Virus (eds Laver, W. G. & Air, G. M.) 1–10 (Elsevier, Amsterdam, 1980).
Min Jou, W. et al. in Structure and Variation in Influenza Virus (eds Laver, W. G. & Air, G. M.) 63–68 (Elsevier, Amsterdam, 1980).
Verhoeyen, M. et al. Nature 286, 771–776 (1980).
Threlfall, G., Barber, C., Carey, N. & Emtage, S. in Structure and Variation in Influenza Virus (eds Laver, W. G. & Air, G. M.) 51–61 (Elsevier, Amsterdam, 1980).
Min Jou, W. et al. Cell 19, 683–696 (1980).
Ward, C. W. & Dopheide, T. A. Biochem. J. (in the press).
Springer, T. A. & Strominger, J. L. Proc. natn. Acad. Sci. U.S.A. 73, 2481–2485 (1976).
Coligan, J. E. et al. Proc. natn. Acad. Sci. U.S.A. 75, 3390–3394 (1978).
Tomita, M. & Marchesi, V. T. Proc. natn. Acad. Sci. U.S.A. 72, 2964–2968 (1975).
Henderson, R. & Unwin, P. N. T. Nature 257, 28–32 (1975).
Engelman, D. M., Henderson, R., McLachlan, A. D. & Wallace, B. A. Proc. Natn. Acad. Sci. U.S.A. 77, 2023–2027 (1980).
Air, G. M. Virology 97, 468–472 (1979).
McCauley, J. et al. FEBS. Lett 108, 422–426 (1979).
Milstein, C., Brownlee, G. G., Harrison, T. M. & Mathews, M. B. Nature new Biol. 239, 117–120 (1972).
Blobel, G. & Dobberstem, B. J. Cell Biol. 67, 835–851 (1975).
Hirst, G. K. J. exp. Med. 75, 49–64 (1942).
Lazarowitz, S. G. & Choppin, P. W. Virology 68, 440–454 (1975).
Klenk. H. D., Rott, R., Orhch, M. & Blodorn, J. Virology 68, 426–439 (1975).
Huang, R. T. C., Wahn, K., Klenk, H. D. & Rott, R. Virology 104, 294–302 (1980).
Wiley, D. C., Wilson, I. A. & Skehel, J. J. Nature 289, 373–378 (1981).
Wiley, D. C. & Skehel, J. J. J. molec. Biol. 112, 343–347 (1977).
Monaco, H. L. thesis, Harvard Uinv. (1978).
Harrison, S. C. J. appl. Crystallogr. l, 84–89 (1968).
Wiley, D. C., Skehel, J. J. & Waterfield, M. D. Virology 79, 446–448 (1977).
Bricogne, G. Acta crystallogr. A32, 832–847 (1976).
Dopheide, T. A. & Ward, C. W. J. gen. Virol. (in the press).
Waterfield, M. D., Scrace, G. & Skehel, J. J. Nature 289, 422–424 (1981).
Harrison, S. C., Olson, A. J., Schutt, C. E., Winkler, F. K. & Bricogne, G. Nature 276, 368–373 (1978).
Abad-Zapatero, C. et al. Nature 286, 33–39 (1980).
Richardson, J. Adv. Protein Chem. (in the press).
Wickner, W. A. Rev. Biochem. 48, 23–45 (1979).
Air, G. M. in Structure and Variation in Influenza Virus (eds Laver, W. G. & Air, G. M.) 135–146 (Elsevier, Amsterdam, 1980).
Pauling, L. & Corey, R. B. Nature 171, 59–61 (1953).
Cohen, C. & Holmes, K. C. J. molec. Biol. 6, 423–432 (1963).
McLachlan, A. D. & Stewart, M. J. molec. Biol. 98, 293–304 (1975).
Ward, C. W. & Dopheide, T. A. Aust. J. biol. Sci. 33, 449–455 (1980).
Deisenhofer, J., Coleman, P. M., Epp, O. & Huber, R. Hoppe-Seyler's Z. physiol. Chem. 357, 1421–1434 (1976).
Marquart, M., Deisenhofer, J., Huber, R. & Palm, W. in Proc. 7th Aharon Kelzir-Katchalsky Conf Ginossar (ed. Balasan, N.) (in the press).
Laver, W. G., Air, G. M., Dopheide, T. A. & Ward, C. W. Nature 283, 454–457 (1980).
Wright, C. S. J. molec. Biol. 141, 267–291 (1980).
Gething, M. J., White, J. M. & Waterfield, M. D. Proc. natn. Acad. Sci. U.S.A. 75, 2737–2740 (1978).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wilson, I., Skehel, J. & Wiley, D. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 Å resolution. Nature 289, 366–373 (1981). https://doi.org/10.1038/289366a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/289366a0
This article is cited by
-
A stapled lipopeptide platform for preventing and treating highly pathogenic viruses of pandemic potential
Nature Communications (2024)
-
Structural characterisation of hemagglutinin from seven Influenza A H1N1 strains reveal diversity in the C05 antibody recognition site
Scientific Reports (2023)
-
Aspects of Biological Replication and Evolution Independent of the Central Dogma: Insights from Protein-Free Vesicular Transformations and Protein-Mediated Membrane Remodeling
The Journal of Membrane Biology (2022)
-
The arrival of highly pathogenic avian influenza viruses H5N8 in Iran through two windows, 2016
Virus Genes (2022)
-
Characterizing genetic and antigenic divergence from vaccine strain of influenza A and B viruses circulating in Thailand, 2017–2020
Scientific Reports (2021)