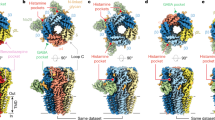
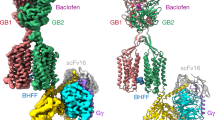
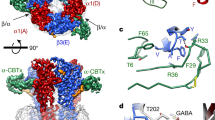
Abstract
The principal inhibitory neurotransmitter GABA (γ-aminobutyric acid) exerts its effects through two ligand-gated channels, GABAA and GABAC receptors, and a third receptor, GABAB (ref. 1), which acts through G proteins to regulate potassium and calcium channels. Cells heterologously expressing the cloned DNA encoding the GABABR1 protein exhibit high-affinity antagonist-binding sites2, but they produce little of the functional activity expected from studies of endogenous GABAB receptors in the brain. Here we describe a new member of the GABAB polypeptide family, GABABR2, that shows sequence homology to GABABR1. Neither GABABR1 nor GABABR2, when expressed individually, activates GIRK-type potassium channels; however, the combination of GABABR1 and GABABR2 confers robust stimulation of channel activity. Both genes are co-expressed in individual neurons, and both proteins co-localize in transfected cells. Moreover, immunoprecipitation experiments indicate that the two polypeptides associate with each other, probably as heterodimers. Several G-protein-coupled receptors (GPCRs) exist as high-molecular-weight species, consistent with the formation of dimers by these receptors3,4,5,6,7, but the relevance of these species for the functioning of GPCRs has not been established. We have now shown that co-expression of two GPCR structures, GABABR1 and GABABR2, belonging to the samesubfamily is essential for signal transduction by GABAB receptors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bowery, N. G. GABABreceptor pharmacology. Annu. Rev. Pharmacol. Toxicol. 33, 109–147 (1993).
Kaupmann, K.et al. Expression cloning of GABABreceptors uncovers similarity to metabotropic gutamate receptors. Nature 386, 239–246 (1997).
Hebert, T. E.et al. Apeptide derived from a beta2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. J. Biol. Chem. 271, 16384–16392 (1996).
Cvejic, S. & Devi, L. A. Dimerization of the delta opioid receptor: implication for a role in receptor internalization. J. Biol. Chem. 272, 26959–26964 (1997).
Maggio, R., Barbier, P., Fornai, F. & Corsini, G. U. Functional role of the third cytoplasmic loop in muscarinic receptor dimerization. J. Biol. Chem. 271, 31055–31060 (1996).
Ciruela, F.et al. Immunological identification of A1 adenosine receptors in brain cortex. J. Neurosci. Res. 42, 818–828 (1995).
Romano, C., Yang, W. L. & O'Malley, K. L. Metabotropic glutamate receptor 5 is a disulfide-linked dimer. J. Biol. Chem. 271, 28612–18616 (1996).
O'Hara, P. J.et al. The ligand-binding domain in metabotropic glutamate receptors is related to bacterial periplasmic binding proteins. Neuron 11, 41–52 (1998).
Bowery, N. G., Hudson, A. L. & Price, G. W. GABAAand GABABreceptor site distribution in the rat central nervous system. Neuroscience 20, 365–383 (1987).
North, R. A. Drug receptors and the inhibition of nerve cells. Br. J. Pharmacol. 98, 13–23 (1989).
Gahwiler, B. H. & Brown, D. A. GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc. Natl Acad. Sci. USA 82, 1558–1562 (1985).
Andrade, R., Malenka, R. C. & Nicoll, R. A. AG protein couples serotonin and GABABreceptors to the same channels in hippocampus. Science 234, 1261–1265 (1986).
Luscher, C., Jan, L. Y., Stoffel, M., Malenka, R. C. & Nicoll, R. A. Gprotein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron 19, 687–697 (1997).
Dascal, N.et al. Atrial G protein-activated K+ channel: expression cloning and molecular properties. Proc. Natl Acad. Sci. USA 90, 10235–10239 (1993).
Krapivinsky, G.et al. The G-protein-gated atrial K channel IKAChis a heteromultimer of two inwardly rectifying K channel proteins. Nature 374, 135–141 (1995).
Kubo, Y., Reuveney, E., Slesinger, P. A., Jan, Y. N. & Jan, L. Y. Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature 364, 802–806 (1993).
Kaupmann, K.et al. Structure, pharmacology and chromosomal localization of GABA-B receptors. Soc. Neurosci. Abstr. 23, 954 (1997).
Habert-Ortoli, E., Amiranoff, B., Loquet, I., Laburthe, M. & Mayaux, J. F. Molecular cloning of a functional human galanin receptor. Proc. Natl Acad. Sci. USA 91, 9780–9783 (1994).
Bon, C. & Galvan, M. Electrophysiological actions of GABABagonists and antagonists in rat dorso-lateral septal neurones in vitro. Br. J. Pharmacol. 118, 961–967 (1996).
Seabrook, G. R., Howson, W. & Lacey, M. G. Electrophysiological characterization of potent agonists and antagonists at pre- and postsynaptic GABABreceptors on neurones in rat brain slices. Br. J. Pharmacol. 101, 949–957 (1990).
Brugger, F., Wicki, U., Olpe, H. R., Froestl, W. & Mickel, S. The action of new potent GABABreceptor angtagonists in the hemisected spinal cord preparation of the rat. Eur. J. Pharmacol. 235, 153–155 (1993).
Avissar, S., Amitai, G. & Sokolovsky, M. Oligomeric structure of muscarinic receptors is shown by photoaffinity labeling: subunit assembly may explain high- and low-affinity agonist states. Proc. Natl Acad. Sci. USA 80, 156–159 (1983).
Wreggett, K. A. & Wells, J. W. Cooperativity manifest in the binding properties of purified cardiac muscarinic receptors. J. Biol. Chem. 270, 22488–22499 (1995).
McLatchie, L. M.et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393, 333–339 (1998).
Nimchinsky, E. A., Hof, P. R., Janssen, W. G. M., Morrison, J. H. & Schmauss, C. Expression of dopamine D3 receptor dimers and tetramers in brain and in transfected cells. J. Biol. Chem. 272, 29229–29237 (1998).
Smith, K. E.et al. Cloned human and rat galanin GALR3 receptors: pharmacology and activation of G-protein inwardly rectifying K+ channels. J. Cell Biol. 273, 23321–23326 (1998).
Durkin, M. M.et al. Localization of messenger RNAs encoding three GABA transporters in rat brain: an in situ hybridization study. Brain Res. Mol. Brain Res. 33, 7–21 (1995).
Quick, M. W. & Lester, H. A. Methods for expression of excitability proteins in Xenopus oocytes. Methods Neurosci. 19, 261–279 (1994).
Salon, J. A. & Owicki, J. C. Real-time measurements of receptor activity: applications of microphysiometric techniques to receptor biology. Methods Neurosci. 25, 201–233 (1995).
Graham, J. in Centrifugation (The Practical Approach Series) (ed. Rickwood, D.) 161–182 (IRL, Oxford, 1984).
Acknowledgements
We thank T. Swayne for use of the confocal microscopy facility at Columbia University; H.-Y. Chang, K. Ogozalek, J. Huang and Y. Wan for support with molecular biology; P. Vaysse for cell biology facilities; C. Forray for advice on membrane fractionation; and S. Rabacchi for helpful hints on protein immunodetection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jones, K., Borowsky, B., Tamm, J. et al. GABAB receptors function as a heteromeric assembly of the subunits GABABR1 and GABABR2. Nature 396, 674–679 (1998). https://doi.org/10.1038/25348
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/25348