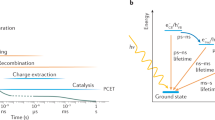
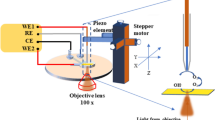
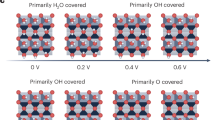
Abstract
ALTHOUGH the possibility of water photolysis has been investigated by many workers, a useful method has only now been developed. Because water is transparent to visible light it cannot be decomposed directly, but only by radiation with wavelengths shorter than 190 nm (ref. 1).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Coehn, A., Ber. Deutsch. Chem. Gesellschaft, 43, 880 (1910).
Fujishima, A., Honda, K., and Kikuchi, S., J. Chem. Soc. Japan (Kogyo Kagaku Zasshi), 72, 108 (1969).
Fujishima, A., and Honda, K., J. Chem. Soc. Japan, 74, 355 (1971).
Fujishima, A., and Honda, K., J. Institute of Industrial Science, University of Tokyo (Seisan Kenkyu), 22, 478 (1970).
Fujishima, A., Sugiyama, E., and Honda, K., Bull. Chem. Soc. Japan, 44, 304 (1971).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
FUJISHIMA, A., HONDA, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 238, 37–38 (1972). https://doi.org/10.1038/238037a0
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1038/238037a0