Abstract
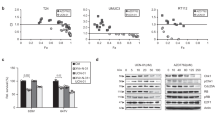
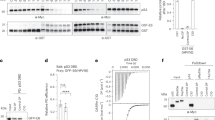
The p53 tumor suppressor protein binds to both cellular and viral proteins, which influence its biological activity. One such protein is the large E1b tumor antigen1 (E1b58kDa) from adenoviruses (Ads), which abrogates the ability of p53 to transactivate various promoters2. This inactivation of p53 function is believed to be the mechanism by which E1b58kDa contributes to the cell transformation process2. Although the p53–E1b58kDa complex occurs during infection3 and is conserved among different serotypes4, there are limited data demonstrating that it has a role in virus replication. However, loss of p53 expression occurs after adenovirus infection of human cells4,5 and an E1b58kDa deletion mutant (Onyx-015, also called dl 1520) selectively replicates in p53-defective cells6,7. These (and other) data indicate a plausible hypothesis is that loss of p53 function may be conducive to efficient adenovirus replication. However, wild-type (wt) Ad5 grows more efficiently in cells expressing a wt p53 protein5. These studies indicate that the hypothesis may be an oversimplification. Here, we show that cells expressing wt p53, as well as p53-defective cells, allow adenovirus replication, but only cells expressing wt p53 show evidence of virus-induced cytopathic effect. This correlates with the ability of adenovirus to induce cell death. Our data indicate that p53 plays a necessary part in mediating cellular destruction to allow a productive adenovirus infection. In contrast, p53-deficient cells are less sensitive to the cytolytic effects of adenovirus and as such raise questions about the use of E1b58kDa-deficient adenoviruses in tumor therapy6,7.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sarnow, P., Ho, Y.S., Williams, J. & Levine, A.J. Adenovirus Elb-58kDa tumor antigen and SV40 large T antigen are physically associated with the same 54kDa protein in transformed cells. Cell 28, 387–394 (1982).
Yew, P.R. & Berk, A.J. Inhibition of p53 transactivation required for transformation by adenovirus early 1b protein. Nature 357, 82–85 ( 1992).
Braithwaite, A.W., Blair, G.E., Nelson, C.C., McGovern, J. & Bellett, A.J.D. Adenovirus Elb-58kDa antigen binds to p53 during infection of rodent cells: evidence for an N-terminal binding site on p53. Oncogene 6, 781– 787 (1991).
Grand, R.J.A., Grant, M.L. & Gallimore, P.H. Enhanced expression of p53 in human cells infected with mutant adenoviruses. Virology 203, 229–240 (1994).
Ridgway, P.J., Hall, A.R., Myers, C.J. & Braithwaite, A.W. p53/E1b58kDa complex regulates adenovirus replication. Virology 237, 404–413 (1997).
Bischoff, J.R. et al. An adenovirus mutant that replicates selectively in p53-deficient tumor cells. Science 274, 373– 376 (1996).
Heise, C. et al. Onyx-015, an E1b gene-attenuated adenovirus, causes tumor -specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nature Med. 3, 639– 645 (1997).
Graham, F.L., Smiley, J., Russell, W.C. & Nairn, R. Characterization of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36, 59– 72 (1977).
Scheffner, M., Munger, K., Byrne, J.C. & Howley, P.M. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc. Natl. Acad. Sci. USA. 88, 5523– 5527 (1991).
Ory, K., LeGros, Y., Auguin, C. & Soussi, T. Analysis of the most representative tumor-derived p53 mutants reveals that changes in protein conformation are not correlated with loss of transactivation or inhibition of cell proliferation. EMBO J. 13, 3496– 3504 (1994).
Philipson, L. & Lindberg, U. in Comprehensive Virology Vol. 3 (eds. Fraenkel-Conrat, H. & Wagner, R.R.) 143– 227 (Plenum, New York, 1973).
Debbas, M. & White, E. Wild-type p53 mediates apoptosis by Ela, which is inhibited by Elb. Genes Dev. 7, 546–554 (1993).
Sharma, S., Schwarte-Waldhoff, I., Oberhuber, H. & Schafer, R. Functional interaction of wild-type and mutant p53 transfected into human tumor cell lines carrying activated RAS genes. Mol. Cell. Biol. 12, 5581–5592 ( 1993).
Slichenmyer, W.J., Nelson, W.G., Slebos, R.J. & Kastan, M.B. Loss of p53-associated G1 checkpoint does not decrease cell survival following DNA damage. Cancer Res. 53, 4164– 4168 (1993).
Anderson, M.J., Casey, G., Fasching, C.L. & Stanbridge, E.J. Evidence that wild-type TP53, and not genes on either chromosome 1 or 11, controls the tumorigenic phenotype of the human fibrosarcoma HT1080. Genes, Chromosomes Cancer 9, 266–281 (1994).
Evdokiou, A. Tumor-suppressive activity of the growth arrest-specific gene, GAS1. Ph.D. thesis, The University of Adelaide (1997).
Dobner, T., Horikoshi, N., Rubenwolf, S. & Shenk, T. Blockage by Adenovirus E4ORF6 of transcriptional activation by the p53 tumor suppressor. Science 272, 1470– 1473 (1996).
Marcellus, R.C. et al. Adenovirus type 5 early region 4 is responsible for E1A-induced p53-independent apoptosis. J. Virol. 70, 6207–6215 (1996).
Lehman, T.A. et al. p53 mutations, ras mutations, and p53-heat shock 70 protein complexes in human lung carcinoma cell lines. Cancer Res. 51, 4090–4096 (1991).
Masuda, H., Miller, C., Koeffler, P.H., Battifora, H. & Cline, M.J. Rearrangement of the p53 gene in human osteogenic sarcoma. Proc. Natl. Acad. Sci. USA. 84, 7716–7719 (1987).
Okamoto, A., et al. Mutations and altered expression of p16ink4 in human cancer. Proc. Natl. Acad. Sci. USA. 91, 11045–11049 (1994).
Romano, J.W. et al. Identification and characterization of a p53 gene mutation in a human osteosarcoma cell line. Oncogene 4, 1483–1488 (1989).
Whitaker, N.J. et al. Involvement of RB-1, p53, p16ink and telomerase in immortalisation of human cells. Oncogene 11, 971–976 (1995).
Ullrich, S.J. et al.Phosphorylation at Ser-15 and Ser-392 in mutant p53 molecules from human tumors is altered compared to wild type p53. Proc. Natl. Acad. Sci. USA 90, 5954–5958 (1993).
Spruck III, C.H. et al. p16 gene in uncultured tumors. Nature 370, 183–184 ( 1994).
Rogan, E.M. et al. Alterations in p53 and p16ink expression and telomere length during spontaneous immortalization of Li-Fraumeni Syndrome Fibroblasts. Mol Cell. Biol. 15, 4745– 4753 (1995).
Pilder, S., Moore, M., Logan, J. & Shenk, T. The adenovirus Elb-58kDa transforming polypeptide modulates transport or cytoplasmic stabilization of viral host cell mRNAs. Mol. Cell. Biol. 6, 470–476 (1986).
Barker, D.D. & Berk, A.J. Adenovirus proteins from both E1b reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology 56, 107– 121 (1987).
Precious, B & Russell, W.C. in Virology: A Practical Approach (ed. Mahy, B.W.J.) 193–205 (IRL, Oxford, England, 1985).
Acknowledgements
We thank A. Berk (UCLA) for dl 1520, T. Shenk (Princeton University) for dl 338, R. Reddel (Sydney, Australia) for III CF/c cells, P. Cowled (Adelaide, Australia) for HT1080 6TGc5 cells and M. Kastan (Johns Hopkins University) for RKO and RKO p53.13 cells. This work was supported by grants to A.B. from the Lottery Board and Cancer Society of New Zealand and the University of Otago Faculty Funds.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hall, A., Dix, B., O'Carroll, S. et al. p53-dependent cell death/apoptosis is required for a productive adenovirus infection. Nat Med 4, 1068–1072 (1998). https://doi.org/10.1038/2057
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/2057