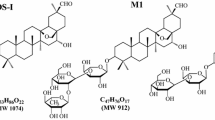
Abstract
Purpose. The purpose of this work was to study the relevant physicochemical properties for the absorption of steroids.
Methods. Various physicochemical properties of steroids were calculated (molecular weight, ClogP, static polar surface area [PSA], etc.). Within this series of steroids, different pharmacological groups were defined. Based on the outcome of this survey, steroids were selected for the Caco-2 permeability study. The apparent permeability coefficients (Papp) were related to the calculated and measured physicochemical properties.
Results. Between the defined groups of steroids, ClogP was the most discriminative descriptor. The steroids were well transported over the cell monolayers and the Papp was independent of the concentration and the transport direction. No relationship was found with the PSA; however, the Papp showed a weak inverse correlation with ClogP.
Conclusions. The molecular descriptors and Papp values showed that all steroids are well transported. The small differences in the Papp values showed a weak inverse correlation with ClogP: the hydrophilic steroids (ClogP approximately 0-2) tend to diffuse faster over the cell monolayers compared with the more hydrophobic steroids (ClogP approximately 5). The relationship with ClogP suggests that partitioning of steroids between the biologic membrane and the surrounding aqueous phase is one of the main mechanisms for absorption.
Similar content being viewed by others
REFERENCES
J. G. Harman and L. E. Limbird. Goodman & Gilman's The Pharmacological Basis of Therapeutics. McGraw Hill, New York, 1996.
U. Norinder, T. Osterberg, and P. Artursson. Theoretical calculation and prediction of Caco-2 cell permeability using MolSurf parameterization and PLS statistics. Pharm. Res. 14:1786-1791 (1997).
M. C. Gres, B. Julian, M. Bourrie, V. Meunier, C. Roques, M. Berger, X. Boulenc, and Y. Berger. Correlation between oral drug absorption in humans, and apparent drug permeability in TC-7 cells, a human epithelial intestinal cell line: comparison with the parenteral Caco-2 cell line. Pharm. Res. 15:726-733 (1998).
M. Yazdanian, S. L. Glyn, J. L. Wright, and A. Hawi. Correlating partitioning and Caco-2 cell permeability of structurally diverse small molecular weight compounds. Pharm. Res. 15:1490-1494 (1998).
S. Yee. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man—fact or myth. Pharm. Res. 14:763-766 (1997).
E. Duizer. Permeability and Modulation of the Tntestinal Epithelial Barrier in Vitro, Ph.D. Thesis, Wageningen Agricultural University, The Netherlands, 1999.
P. Schmiedlin-Ren, K. E. Thummel, J. M. Fischer, M. F. Paine, K. S. Lown, and P. B. Watkins. Expression of enzymatically active CYP3A4 by Caco-2 cells grown on extracellular matrix-coated permeable supports in the presence of 1alpha,25-dihydroxyvitamin D3. Mol. Pharmacol. 51:741-754 (1997).
S. D. Raeissi, I. J. Hidalgo, J. Segura-Aguilar, and P. Artursson. Interplay between CYP3A-mediated metabolism and polarized efflux of terfenadine and its metabolites in intestinal epithelial Caco-2 (TC7) cell monolayers. Pharm. Res. 16:625-632 (1999).
F. Labrie, V. Luu-The, and S. X. Lin. The key role of 17beta-hydroxysteroid dehydrogenases in sex steroid biology. Steroids 62:148-158 (1997).
S. Andersson and N. Moghrabi. Physiology and molecular genetics of 17beta-hydroxysteroid dehydrogenases. Steroids 62:143-147 (1997).
J. Kelder, P. D. Grootenhuis, D. M. Bayada, L. P. Delbressine, and J. P. Ploemen. Polar molecular surface as a dominating determinant for oral absorption and brain penetration of drugs. Pharm. Res. 16:1514-1519 (1999).
F. J. Zeelen. Medicinal Chemistry of Steroids, Elsevier Science Publishers BV, Amsterdam, 1990.
U. Kragh-Hansen. Molecular aspects of ligand binding to serum albumin. Pharmacol. Rev. 33:17-53 (1981).
S. Watanabe and T. Sato. Effects of free fatty acids on the binding of bovine and human serum albumin with steroid hormones. Biochim. Biophys. Acta 1289:385-396 (1996).
M. E. Baker. Albumin's role in steroid hormone action and the origin of vertebrates: is albumin an essential protein? FEBS Lett. 439:9-12 (1998).
J. M. Fischer, S. A. Wrighton, J. C. Calamia, D. D. Shen, and K. L. Kunze. Midazolam metabolism by modified Caco-2 monolayers: effects of extracellular protein binding. J. Pharmacol. Exp. Ther. 289:1143-1150 (1999).
S. Yamashita, T. Furubayashi, M. Kataoka, T. Sakane, H. Sezaki, and H. Tokuda. Optimized conditions for prediction of intestinall drug permeability using Caco-2 cells. Eur. J. Pharm. Sci. 10:195-204 (2000).
C. A. Lipinski, F. Lombardo, B. W. Dominy, and P. J. Feeny. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug. Deliv. Rev. 23:3-25 (1997).
A. Lampen, A. Bader, T. Bestmann, M. Winkler, L. Witte, and J. T. Borlak. Catalytic activities, protein-and mRNA-expression of cytochrome P450 isoenzymes in intestinal cell lines. Xenobiotica 28:429-441 (1998).
T. Prueksaritanont, L. M. Gorham, J. H. Hochmann, L. O. Tran, and K. P. Vyas. Comparative studies of drug-metabolizing enzymes in dog, monkey, and human small intestines, and in Caco-2 cells. Drug Metab. Dispos. 24:634-642 (1996).
C. H. M. Versantvoort, R. C. A. Onderwater, E. Duizer, J. J. M. Van de Sandt, A. J. Gilde, and J. P. Groten. Monolayers of IEC-18 cells as an in vitro model for screening the passive transcellular and paracellular transport across the intestinal barrier: comparison of active and passive transport with the human colon carcinoma Caco-2 cell line. Environ. Toxicol. Pharmacol. 11:335-344 (2002).
R. Sandstrom, A. Karlsson, L. Knutson, and H. Lennernas. Jejunal absorption and metabolism of R/S verapamil in humans. Pharm. Res. 15:856-862 (1998).
G. Ecker, M. Huber, D. Schmid, and P. Chiba. The importance of a nitrogen atom in modulators of multidrug resistance. Mol. Pharmacol. 56:791-796 (1999).
S. Ernest and E. Bello-Reuss. P-glycoprotein functions and substrates: possible roles of MDR1 gene in the kidney. Kidney Int. 65(Suppl):S11-S17 (1998).
K. M. Barnes, B. Dickstein, G. B. Cutler Jr., T. Fojo, and S. E. Bates. Steroid transport, accumulation, and antagonism of P-glycoprotein in multidrug resistant cells. Biochemistry 35:4820-4827 (1996).
G. Deliconstantinos and S. Fotiou. Sex steroid and prostaglandin interactions upon the purified rat myometrial plasma membranes. Mol. Cell. Endocrinol. 45:149-156 (1986).
H. Saitoh, M. Hatakeyama, O. Eguchi, M. Oda, and M. Takada. Involvement of intestinal P-glycoprotein in the restricted absorption of methylprednisolone from rat small intestine. J. Pharm. Sci. 87:73-75 (1998).
K. A. Lentz, J. W. Polli, S. A. Wring, J. E. Humphreys, and J. E. Polli. Influence of passive permeability on apparent P-glycoprotein kinetics. Pharm. Res. 17:1456-1460 (2000).
S. Winiwarter, N. M. Bonham, F. Ax, A. Hallberg, H. Lennernas, and A. Karlen. Correlation of human jejunal permeability (in vivo) of drugs with experimentally and theoretically derived parameters. A multivariate data analysis approach. J. Med. Chem. 41:4939-4949 (1998).
T. I. Oprea and J. Gottfries. Toward minimalistic modeling of oral drug absorption. J. Mol. Graphics Mod. 17:261-274 (1999).
H. van de Waterbeemd, G. Camenisch, G. Folkers, and O. A. Raevsky. Estimation of Caco-2 cell permeability using calculated molecular descriptors. Quant. Struct. Act. Relat. 15:480-490 (1996).
L. Stryer. Biochemistry, W.H. Freeman and Company, New York, 1995.
H. R. Lamche, P. T. Silberstein, A. C. Knabe, D. D. Thomas, H. S. Jacob, and D. E. Hammerschmidt. Steroids decrease granulocyte membrane fluidity, while phorbol ester increases membrane fluidity. Studies using electron paramagnetic resonance. Inflammation 14:61-70 (1990).
V. B. Mahesh, D. W. Brann, and L. B. Hendry. Diverse modes of action of progesterone and its metabolites. J. Steroid Biochem. Mol. Biol. 56:209-219 (1996).
G. A. Golden, R. P. Mason, T. N. Tulenko, G. S. Zubenko, and R. T. Rubin. Rapid and opposite effects of cortisol and estradiol on the human erythrocyte Na+,K+-ATPase activity: relationship to steroid intercalation into the cell membrane. Life Sci. 65:1247-1255 (1999).
D. B. Goldstein. The effects of drugs on membrane fluidity. Annu. Rev. Pharmacol. Toxicol. 24:43-64 (1984).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Faassen, F., Kelder, J., Lenders, J. et al. Physicochemical Properties and Transport of Steroids Across Caco-2 Cells. Pharm Res 20, 177–186 (2003). https://doi.org/10.1023/A:1022210801734
Issue Date:
DOI: https://doi.org/10.1023/A:1022210801734