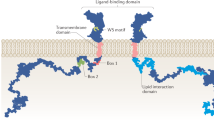
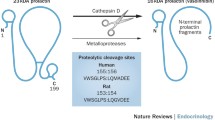
Abstract
Prolactin (PRL) regulates the development of themammary gland at three stages in the reproductive lifehistory of females. The first stage is mammary glandorganogenesis, during which PRL contributes to the maturation of the mammary glands from aprimary ductal system, which grows from terminal endbuds, to the fully mature nonpregnant gland. The maturemammary gland is characterized by an absence of terminal end buds, and the development of a highlybranched architecture, which is decorated by lobularbuds. During pregnancy PRL, placental lactogens, andprogesterone stimulate the expansion and physiological differentiation of the lobuloalveolar systemfrom the lobular buds. After delivery PRL, in thecontext of falling progesterone, stimulates the finalinduction of milk protein gene expression and lactation. PRL acts directly on the mammary epithelium,and indirectly by stimulating luteal progesteronesecretion in rodents. Disruption of the genes for PRLand the PRL receptor, as well as those for transcription factors important in mammary gland regulation(Stat proteins), have provided a new set of animalmodels with which to study normal mammary glanddevelopment and the relationships of PRL to breastcarcinogenesis. Two major deficiencies in our current knowledgeof PRL actions are our understanding of the role ofepithelial-stromal interactions in PRL-induced mammarymorphogenesis, and the identity of developmentally important genes that are regulated by PRLduring normal mammary gland organogenesis.
Similar content being viewed by others
REFERENCES
S. Swanson, R. Guzman, K. Christov, S. Miyamoto, and S. Nandi (1994). Pituitary-isografted mice are highly susceptible to MNU-induced mammary carcinogensis irrespective of the level of alveolar differentiation. Carcinogenesis 15: 1341–1346.
O. Riddle, R.W. Bates, and S.W. Dykshorn (1933). The preparation, identification and assay of prolactin-a hormone of the anterior pituitary. Am. J. Physiol. 105:191–216.
Y.J. Topper and C.S. Freeman (1980). Multiple hormone interactions in the developmental biology of the mammary gland. Physiol. Rev. 60:1049–1106.
C.S. Nicoll (1980). Prolactin: Ontogeny and evolution of prolactin's functions. Fed. Am. Soc. Exp. Biol. 39:2561–2566.
S.L. Frawley (1989). Mammosomatotropes: Current status and possible functions. Trends Endocrinol. Metabol. 1:31–34.
N. Ben-Jonathan, J.L. Mershon, D.L. Allen, R.W. Steinmets (1997). Extrapituitary prolactin: Distribution, regulation, functions, and clinical aspects. Endocrine Rev. 17:639–669.
M. Kelly, M. Rubinstein, S. Asa, G. Zhang, C. Saez, J. Bunzow, R. Allen, R. Hnasko, N. Ben-Jonathan, D. Grandy, and M. Low (1997). Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron 19:103–113.
M.A. Seyfred and J. Gorski (1990). An interaction between the 5′ flanking distal and proximal regulatory domains of the rat prolactin gene is required for transcriptional activation by estrogens. Mol. Endocrinol. 4:1226–1234.
G.-z. Yan, W.T. Pan, and C. Bancroft (1991). Thyrotropin-releasing hormone action on the prolactin promoter is mediated by the POU protein Pit-1. Mol. Endocrinol. 5:535–541.
S. Bredow, B. Kacsóh, F. Obál, Jr., J. Fang, and J. M. Krueger (1994). Increase of prolactin mRNA in the rat hypothalamus after intracerebroventricular injection of VIP or PACAP. Brain Res. 660:301–308.
C. A. Pickett and A. Gutierrez-Hartmann (1994). Ras mediates Src but not epidermal growth factor-receptor tyrosine kinase signaling pathways in GH4 neuroendocrine cells. Proc. Natl. Acad. Sci. U.S.A. 91:8612–8616.
T.E. Porter, C.D. Wiles, and L.S. Frawley (1994). Stimulation of lactotrope differentiation in vitro by fibroblast growth factor. Endocrinology. 134:164–168.
N. Ben-Jonathan, L.A. Arbogast, and J.F. Hyde (1989). Neuroendocrine regulation of prolactin release. Prog. Neurobiol. 33:399–447.
R. W. Steger, V. Chandrashekar, W. Zhao, A. Bartke, and N. D. Horseman (1998). Neuroendocrine and reproductive functions in male mice with targeted disruption of the prolactin gene. Endocrinology 139:3691–3695.
M.J. Soares, H. Muller, K.E. Orwig, T.J. Peters, and G. Dai (1998). Uteroplacental prolactin family and pregnancy. Biol. Repro. 58:273–284.
S. Handwerger (1991). Clinical counterpoint: The physiology of placental lactogen in human pregnancy. Endocrine Rev. 12:329–336.
B. Gellerson, G.E. Dimattia, H.G. Friesen, and H.G. Bohne (1989). Prolactin (PRL) mRNA from human decidua differs from pituitary PP mRNA but resembles the IM-9–P3 lymphoblast PRL transcript. Mol. Cell Endocrinol. 64:127–130.
B. Kacsóh, Z. Veress, B.E. Tóth, L.M. Avery, and C.E. Grosvenor (1993). Bioactive and immunoreactive variants of prolactin in milk and serum of lactating rats and their pups. J. Endocrinol. 138:243–257.
I. A. Forsyth (1982). Oxford Reviews of Reproductive Biology, Clarendon Press, Oxford.
L. Hennighausen, G.W. Robinson, K.-U. Wagner, and X. Liu (1997). Prolactin signaling in mammary gland development. J. Biol. Chem. 272:1–xxx.
N.E. Hynes, N. Cella, and M. Wartmann (1997). Prolactin mediated intracellular signaling in mammary epithelial cells. J. Mam. Gland Biol. Neoplasia 2:19–27.
C.J. Ormandy and R.L. Sutherland (1993). Mechanisms of prolactin receptor regulation in mammary gland. Mol. Cell. Endocrinol. 91:C1–C6.
I.H. Russo, M. Tewaro and J. Russo. (1989). Morphology and development of the rat mammary gland. Integument Mam. Glands pp. 233–252.
R.W. Turkington, G.C. Majumder, N. Kadohama, J.H. MacIndoe, and W.L. Frantz (1973). Hormonal regulation of gene expression in mammary cells. J. Rec. Horm. Res. 29:417–455.
B.K. Vonderhaar (1985). Control of Cell Growth and Proliferation, Van Nostrand Reinhold Inc., Stroundsburg, Pennsylvania.
J.J. Wysolmerski, J.F. McCaughern-Carucci, A.G. Daifotis, A.E. Broadus, and W.M. Philbrick (1995). Overexpression of parathyroid hormone-related protein or parathyroid hormone in transgenic mice impairs branching morphogenesis during mammary gland development. Development 121:3539–3547.
M.E. Dunbar and J.J. Wysolmerski (1999). Parathyroid hormone-related protein: A developmental regulatory molecule necessary f. mammary gland development J. Mam. Gland Biol. Neoplasia 4(1):xxx-xxx.
D.L. Kleinberg (1997). Early mammary development: Growth hormone and IGF-1. J. Mam. Gland Biol. Neoplasia 2:49–57.
M. Schmitt-Ney, B. Happ, P. Hofer, N.E. Hynes, and B. Groner (1992). Mammary gland-specific nuclear factor activity is positively regulated by lactogenic hormones and negatively by milk stasis. Mol. Endocrinol. 6:1988–1997.
W. Lyons, C.H. Li, and R.E. Johnson (1958). Hormonal control of mammary growth and lactation. Recent Prog. Horm. Res. 14:219–254.
N.D. Horseman, W. Zhao, E. Montecino-Rodriguez, M. Tanaka, K. Nakashima, S.J. Engle, F. Smith, E. Markoff, and K. Dorshkind (1997). Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J. 16:6926–6935.
C. Ormandy, J., A. Camus, J. Barra, D. Damotte, B. Lucas, H. Buteau, M. Edery, N. Brousse, C. Babomet, N. Binart, and P.A. Kelly (1997). Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 11:167–178.
G. Chepko and G.H. Smith (1997). Three division-competent, structurally-distinct cell populations contribute to murine mammary epithelial renewal. Tissue Cell 29:239–253.
G.H. Smith (1996). Experimental mammary epithelial morphogenesis in an in vivo model: Evidence for distinct cellular progenitors of the ductal and lobular phenotype. Breast Cancer Res. Treat. 39:21–31.
G. Chepko and G.H. Smith (1999). Mammary epithelial slew cells: Our current understanding. J. Mam. Gland Biol. Neoplasia 4(1):xxx-xxx.
C.T. Albarracin, T.G. Parmer, W.R. Duan, S.E. Nelson, and G. Gibori (1994). Identification of a major prolactin-regulated protein as 20α-hydroxysteroid dehydrogenase: Coordinate regulation of its activity, protein content, and messenger ribonucleic acid expression. Endocrinology 134:2453–2460.
S. Haslam (1988). Acquisition of estrogen-dependent progesterone receptors by normal mouse mammary gland. Ontogeny of mammary progesterone receptors. J. Steroid Biochem. 31:9–13.
C.J. Ormandy, J. Graham, P.A. Kelly, C.L. Clarke, and R. L. Sutherland (1992). Effects of progestins on prolactin receptor gene transcription in human breast cancer cells. DNA Cell Biol. 11:721–726.
S. Wang, L.J. Counterman, and S.Z. Haslam (1990). Progesterone action in normal mouse mammary gland. Endocrinology 127:2183–2189.
F.E. Jones, D.J. Jerry, B.C. Guarino, G.C. Andrews, and D.F. Stern (1996). Heregulin induces in vivo proliferation and differentiation of mammary epithelium into secretary lobuloalveoli. Cell Growth Differ. 7:1031–1038.
I.M. Krane and P. Leder (1996). NDF/heregulin induces persistence of terminal end buds and adenocarcinomas in the mammary glands transgenic mice. Oncogene 12:1781–1788.
Y. Yang, E. Spitzer, D. Meyer, M. Sachs, C. Niemann, G. Hartmann, K.M. Weidner, C. Birchmeier, and W. Birchmeier (1995). Sequential requirement of hepatocyte growth factor and neuregulin in the morphogenesis and differentiation of the mammary gland. J. Cell Biol. 131:215–226.
B.M. Marte, M. Jeschke, D. Graus-Porta, D. Taverna, P. Hofer, B. Groner, Y. Yarden, and N.E. Hynes (1995). Neu differentiation factor/heregulin modulates growth and differentiation of HC11 mammary epithelial cells. Mol. Endocrinol. 9:14–23.
P. Sicinski, J.L. Donaher, S.B. Parker, T. Li, A. Fazeli, H. Gardner, S.Z. Haslam, R.T. Bronson, S.J. Elledge, and R.A. Weinberg (1995). Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 82:621–630.
J.P. Lydon, F.J. DeMayo, C.R. Funk, S.K. Mani, A.R. Hughes, C.A. Montgomery, Jr., G. Shyamala, O.M. Conneely, and B.W. O'Malley (1995). Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Gene Dev. 9:2266–2278.
J.M. Rosen, S.L.C. Woo, and J.P. Comstock (1975). Regulation of casein messenger RNA during the development of the rat mammary gland. Biochemistry 14:2895–2903.
S.C. Supowit and J.M. Rosen (1980). Gene expression in normal and neoplastic mammary tissue. Biochemistry 19:3452–3460.
L.-y. Yu-Lee and J.M. Rosen (1988). A transfected alpha-casein minigene bypasses posttranscriptional control by hormones, but retains cell-substratum regulation in mammary epithelial cells. Mol. Endocrinol. 2:431–443.
P. Poyet, S.J. Henning, and J.M. Rosen (1989). Hormone-dependent β-casein mRNA stabilization requires ongoing protein synthesis. Mol. Endocrinol. 3:1961–1968.
A. Baruch, M. Shani, and I. Barash (1998). Insulin and prolactin synergize to induce translation of human serum albumin in the mammary gland pf transgenic mice. Transgenic Res. 7:15–27.
P.A. Kelley, S. Ali, M. Rozakis, L. Goujon, M. Nagano, I. Pellegrini, D. Gould, J. Djiane, M. Edery, J. Finidori, and M.C. Postel-Vinay (1993). The growth hormone/prolactin receptor family. Rec. Prog. Horm. Res. 48:123–164.
V. Goffin and P.A. Kelly. (1997). The prolactin/growth hormone receptor family: Structure/function relationships. J. Mam. Gland Biol Neoplasia 2:7–17.
C. Bole-Feysot, V. Goffin, M Edery, N Binart, and P.A. Kelly (1998). Prolactin (PRL) and its receptor: Actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocrine Rev. 19:225–268.
N.D. Horseman and L.-y. Yu-Lee (1994). Transcriptional regulation by the helix bundle peptide hormones: GH, PRL, and hematopoietic cytokines. Endocrine Rev. 15:627–649.
D. Cosman, S.D. Lyman, R.L. Idzerda, M.P. Beckmann, L.S. Park, R.G. Goodwin, and C.J. March. (1990). A new cytokine receptor superfamily. Trends Biochem. Sci. 15:265–270.
J.E. Darnell, Jr., I.M. Kerr, and G.R. Stark (1994). Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264:1415–1421.
X. Liu, G.W. Robinson, and L. Henninghausen (1996). Activation of Stat5a and Stat5b by tyrosine phosphorylation is tightly linked to mammary gland differentiation. Mol. Endocrinol. 10:1496–1506.
Y.-F. Wang and L.-Y. Yu-Lee (1996). Multiple Stat complexes interact at the interferon regulatory factor-1 interferon-gamma activation sequence in the prolactin-stimulated Nb2 T cells. Mol. Cell Endocrinol. 121:19–28.
X. Liu, G.W. Robinson, K.-U. Wagner, L. Garrett, A. Wynshaw-Boris, and L. Hennighausen (1997). Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 11:179–186.
G.B. Udy, R.P. Towers, R.G. Snell, R.J. Wilkins, S.-H. Park, P.A. Ram, D.J. Waxman, and H. W. Davey (1997). Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc. Natl Acad. Sci. U.S.A.94:7239–7244.
S. Teglund, C. McKay, E. Schuetz, J.M. van Deursen, D. Stravopodis, D. Wang, M. Brown, S. Bodner, G. Grosveld, and J.N. Ihle (1998). Stat5a and Stat5b proteins have essential and nonessential, or redundnat, roles in cytokine responses. Cell 93:841–850.
C.M. Shaw-Bruha, S.J. Pirrucello, and J.D. Shull (1997). Expression of the prolactin gene in normal neoplastic human breast tissue and human mammary cell lines: Usage and alternative mRNA splicing. Breast Cancer Res. Treat. 44:243–253.
C.V. Clevenger and M.V. Medaglia (1994). The protein tyrosine kinase p59fyn is associated with prolactin (PRL) receptor and is activated by PRL stimulation of T-lymphocytes. Mol. Endocrinol. 8:674–681.
Rights and permissions
About this article
Cite this article
Horseman, N.D. Prolactin and Mammary Gland Development. J Mammary Gland Biol Neoplasia 4, 79–88 (1999). https://doi.org/10.1023/A:1018708704335
Issue Date:
DOI: https://doi.org/10.1023/A:1018708704335