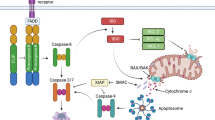
Abstract
Tumor necrosis factor-α (TNF-α), a proinflammatory cytokine, exerts its biological activity by signaling via its two receptors, TNF-RI and TNF-RII, and by activating NF-κB. NF-κB is essential for survival of many cell types; however, TNF-α also induces cell death. In this article, both the survival and cell death signaling by TNF-α and the role of caspases in turning off NF-κB survival signal are reviewed. Furthermore, a role of DAP kinase in TNF-induced apoptosis is discussed. Finally, the molecular basis of the effect of age on TNF-α-induced apoptosis in human T cells is reviewed.
Similar content being viewed by others
REFERENCES
Gupta S: Molecular steps of TNF receptor-mediated apoptosis. Curr Mol Med 1:299–306, 2001
Ashkanazi A, Dixit VM: Death receptors: Signaling and modulation. Science 281:1305–1308, 1998
Screaton G, Xu X-N: T cell life and death signaling via TNFreceptor family members. Curr Opin Immunol 12:316–322, 2000
Tartaglia LA, Goeddel DV: Two TNF receptors. Immunol Today 13:151–153, 1992
Vandenabeele P, Declercq W, Beyaert R, Fiers W: Two tumor necrosis factor receptors: Structure and function. Trends Cell Biol 5:392–399, 1995
Thomas B, Grell M, Pfizenmaier K, Scheurich P: Identification of a 60-kDa tumor necrosis factor (TNF) receptor as the major signal transducing component in TNF responses. J Exp Med 172:1019–1023, 1990
Darnay BG, Aggarwal BB: Early events in TNF signaling: a story of associations and dissociations. J Leukocyte Biol 61:559–566, 1997
Yuan J: Transducing signals of life and death. Curr Opinion Cell Biol 9:247–251, 1997
Rothe J, Gehr G, Loetcher H, Lesslauer W: Tumor necrosis factor receptor-structure and function. Immunol Res 11:81–90, 1992
Wallach D: Suicide by order: Some open questions about the cell-killing activities of the TNF ligand and receptor families. Cytok Growth Factor Rev 7:211–221, 1996
Wallach D, Boldin M, Varfolomeev E, Beyaert R, Vandenabeele P, Fiers W: Cell death induction by receptors of the TNF family: Towards a molecular understanding. FEBS Lett 410:96–106, 1997
Fagiola U, Cossarizza A, Scala E, Fanales-Belasio E, Ortolani C, Cozzi E, Monti D, Franceschi C, Paganelli R: Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol 23:2375–2378, 1993
Aggarwal S, Gollapudi S, Gupta S: Increased TNF-?-induced apoptosis in lymphocytes from aged humans: Changes in TNF-? receptor expression and activation of caspases. J Immunol 162:2154–2161, 1999
Gupta S: Molecular steps of death receptor and mitocondrial pathways of apoptosis. Life Sci 69:2957–2964, 2001
Gupta S: Molecular and biochemical pathways of apoptosis in lymphocytes from aged humans. Vaccine 18:1596–1601, 2000
Gupta S: Molecular steps of cell suicide: An insight into immune senescence. J Clin Immunol 20:229–239, 2000
Hu S, Vincenz C, Ni J, Gentz R, Dixit VM: I-Flice, a novel inhibitor of tumor necrosis factor receptor-1 and CD95-induced apoptosis. J Biol Chem 272:17255–17257, 1997
Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer J-L, Schriter M, Burns K, Mattmann C, Rimoldi D, French LE, Tschopp J: Inhibition of death receptor signals by cellular FLIP. Nature 388:190–195, 1997
Hu S, Vincenz C, Buller M, Dixit VM: A novel family of viral death effector domain-containing molecules that inhibit both CD95-and tumor necrosis factor receptor-1-induced apoptosis. J Biol Chem 272:9621–9624, 1997
Kataoka T, Schroter M, Hahne M, Schneider P, Irmler M, Thome M, Froelich CJ, Tschopp J: FLIP prevents apoptosis induced by death receptors but not by perforin/granzyme B, chemotherapeutic drugs, and gamma irradiation. J Immunol 161:3936–3942, 1998
Kataoka T, Budd RC, Holler N, Thome M, Martinon F, Irmler M, Burns K, Hahne M, Kennedy N, Kovascovics M, Tschopp J: The caspase-8 inhibitor FLIP promotes activation of NF-КB and Erk signaling pathways. Curr Biol 10:640–648, 2000
Liu ZG, Hsu H, Goeddel DV, Karin M: Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappa-B activation prevents cell death. Cell 87:565–576, 1996
Beg AA, Baltimore D: An essential role for NF-КB in preventing TNF-?-induced cell death. Science 274:782–784, 1996
Natoli G, Costanzo A, Lanni A, Templeton DJ, Woodgett JR, Balsano C, Levero M: Activation of SAPK/JNK by TNF receptor 1 through a noncytotoxic TRAF-2-dependent pathway. Science 275:200–203, 1997
Ichijo N, Nishida E, Irie K, Ten Dijke P, Saitoh M, Moriguchi T, Tagaki M, Matsumoto K, Miyazono K, Gotoh Y: Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275:90–94, 1997
Shan R, Price JO, Gaarde WA, Monia BP, Krantz SB, Zhao ZJ: Distinct roles of JNKs/p38 MAP kinase and ERKs in apoptosis and survival of HCD-57 cells induced by withdrawal of addition of erythropoietin. Blood 94:4067–4076, 1999
Zeuner A, Ricci-Vitiani L, Conticello C, De Mari R: Death in 2000 ways. Cell Death Diff 7:1140–1144, 2000
Lin Y, Devin A, Rodriguez Y, Liu ZG: Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev 13:2514–2526, 1999
Deveraux QL, Stennicke HR, Salvesen GS, Reed JC.: Endogenous inhibitors of caspases. J. Clin. Immunol 19:350–364, 1999
Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV: The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell 83: 1243–1252, 1995
Weiss T, Grell M, Siekienski K, Muhlenbeck F, Durkop H, Pfizenmaier K, Scheurich P, Wajant H: TNFR80-dependent enhancement of TNFR60-induced cell death is mediated by TNFR-associated factor 2 and is specific for TNFR60. J Immunol 161:3136–3142, 1998
Declercz W, Denecker G, Fiers W, Vandenabeele P: Cooperation of both TNF receptors in inducing apoptosis: Involvement of the TNF receptor-associated factor binding domain of the TNF receptor 75. J Immunol 161:390–399, 1998
Haridas V, Darnay BG, Natrajan K, Helle R, Aggarwal BB: Overexpression of the p80 TNFR leads to TNF-dependent apoptosis, nuclear factor-kappa B activation. J Immunol 160:3152–3162, 1998
Vandenabeele P, Declercq W, Vanhaesebroeck B, Grooten J, Fiers W: Both TNF receptors are required for TNF-mediated induction of apoptosis in PC60 cells. J Immunol 154:2904–2913, 1995
Weiss T, Grell M, Siekienski K, Muhlenbeck F, Durkop H, Pfizenmaier K, Scheurich P, Wajant HJ: TNFR-80-dependent enhancement of TNFR60-induced cell death is mediated by TNFR-associated factor 2 and is specific for TNFR60. J Immunol 161:3136–3142, 1998
Tartaglia L, Pennica D, Goddel DV: Ligand passing: the 75-kDa tumor necrosis factor (TNF) receptor recruits TNF for signaling by the p55-kDa TNF receptor. J Biol Chem 268:18542–18548, 1993
Zheng L, Fisher G, Miller RE, Peschn J, Lynch DH, Lenardo MJ: Induction of apoptosis in mature T cells by tumor necrosis factor. Nature 377:348–351, 1995
Grell M, Zimmermann G, Gottfried E, Chen CM, Grunwald U, Huang DC, Wu Lee YH, Durkop H, Englemann H, Scheurich P: Induction of cell death by tumor necrosis factor (TNF) receptor 2, CD40, and CD30: A role of TNFR1 activation by endogenous membrane-anchored TNF. EMBO J 18:3034–3043, 1999
Pimentel-Muinos FX, Seed B: Regulated commitment of TNF receptor signaling: A molecular switch for death or activation. Immunity 11:783–793, 1999
Kelliher MA, Grimm S, Ishida Y, Kuo F, Stranger BZ, Leder P: The death domain kinase RIP mediates the TNF-induced NFkappa B signal. Immunity 8:297–303, 1998
Chen FK-M, Lenardo, MJ: A crucial role for p80 TNF-R2 in amplifying p60 TNF-RI apoptosis signals in T lymphocytes. Eur J Immunol 30:652–660, 2000
Baeuerle PA, Henkel T: Function and activation of NF-КB in the immune system. Ann Rev Immunol 12:141–179, 1994
Ghosh S, May MJ, Kopp EB: NF-КB and rel proteins: Evolutionarily conserved mediators of immune responses. Annu Rev Immunol 16:225–260, 1998
Baldwin AS: The NF-КB and IКB proteins: New discoveries and insights. Annu Rev Immunol 14:649–681, 1996
Brown K, Gerstberger, S Carlson, L Franzoso, G Siebenlist, U: Control of IКB-? proteolysis by site-specific, signal induced phosphorylation. Science 281:1360–1363, 1995
Delhase M, Hayakawa M, Chen Y, Karin M: Positive and negative regulation of IКB kinase activity through IKK? subunit phosphorylation. Science 284:309–313, 1999
Zandi E, Chen YI, Karin M: Direct phosphorylation of IКB by IKK? and IKK?: Discrimination between free and NF-КB-bound substrate. Science 281:1360–1363, 1998
Pahl HL: Activators and target genes of Rel/NF-kB transcription factors. Oncogene 18:6855–6866, 1999
Karin M: How NF-КB is activated: The role of the IkB kinase (IKK) complex. Oncogene 18:6867–6874, 1999
Karin M, Lin A: NF-КB at the crossroads of life and death. Nature Immunol 3:221–227, 2002
Liu ZG, Hsu H, Goeddel DV, Karin M: Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappa-B activation prevents cell death. Cell 87:565–576, 1996
Li ZW, Chu WM, Hu YL, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M: The IKKβ subunit of IКB kinase (IKK) is essential for nuclear factor-КB activation and prevention of apoptosis. J Exp Med 189:1839–1845, 1999
Senftleben U, Li Z-W, Baud V, Karin M: IKKβ is essential for protecting T cells from TNFα-induced apoptosis. Cell 14:217–230, 2001
Beg A, Sha WC, Bronson RT, Ghosh S, Baltimore D: Embryonic lethality and liver degeneration in mice acking the RelA component of NF-КB. Nature 376:167–170, 1995
Li Q, Antwerp DV, Mercurio F, Lee K-F, Verma IM: Severe liver degeneration in mice lacking the IКB kinase 2 gene. Science 284:321–324, 1999
Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM, Alnemri ES, Salvesen GS, Reed JC: IAPs block apoptotic events induced by caspase-8 and cytocrome c by direct inhibition of distinct caspases. EMBO J 17:2215–2223, 1998
Hong SY, Yoon WH, Park JH, Kang SG, Ahn JH, Lee TH: Involvement of two NF-КB binding elements in TNF-α, CD40, and Epstein-Barr virus latent membrane protein 1-mediated induction of inhibitor of apoptosis protein 2 gene. J Biol Chem 275:18022–18028, 2000
Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS Jr: NF-КB antiapoptosis: Induction of TRAF1 and TRAF2 and cIAP1 and cIAP2 to suppress caspase-8 activation. Science 281:1680–1683, 1998
Liston P, Roy N, Tamai K, Lefebvre C, Baird S: Suppression of apoptosis in mammalian cells by NIAP and a related family of IAP genes. Nature 379:349–353, 1996
Stehlik C, de Martin R, Kumabashiri I, Schmid JA, Binder BR, Lipp J: NF-КB-regulated xiap gene expression protects endothelial cells from TNF-α-induced apoptosis. J Exp Med 188:211–216, 1998
Takahashi R, Deveraux Q, Tamm I, Welsh K, Assa-Munt N, Salvesen GS, Reed JC: A single BIR domain of XIAP sufficient for inhibiting caspases. J Biol Chem 273:7787–7790, 1998
Tang G, Minemoto, Y, Dibling, B, Purcell, NH, Li, Z, Karin, M, Lin, A: Inhibition of JNK activation through NF-КB target genes. Nature 414:313–317, 2001
De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Cong R, Franzoso G: Induction of gadd45β by NF-КB down-regulates proapoptotic JNK signaling. Nature 414:308–313, 2001
Fomace AJJ, Jackman J, Hollander MC, Hoffman-Liebermann B, Lieberman DA: Genotoxic stress-response genes and growth arrest genes. Gad, MyD, and other genes induced by treatments eliciting growth arrest. Ann New York Acad Sci 663:139–153, 1992
Lin EY, Orlofsky A, Berger MS, Prystowsky MB: Characterization of A1, a novel hematopoietic-specific early-response gene with sequence similarity to bcl-2. J Immunol 151:1979–1988, 1993
Zong WX, Edelstein LC, Chen C, Bash J, Gelinas C: The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-КB that blocks TNF-α-induced apoptosis. Genes Dev 13:382–387, 1999
Lee HH, Dadgostart H, Cheng Q, Shu J, Cheng G: NF-КBmediated up-regulation of Bcl-X and Bfl-1/A1 is required for CD40 survival signaling in B lymphocytes. Proc Natl Acad Sci USA 99:9136–9141, 1999
Wang CY, Guttridge DC, Mayo MW, Baldwin AS Jr: NF-КB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol Cell Biol 19:5923–5929, 1999
Tamatani M, Che YH, Matsuzaki H, Ogawa S, Okado H, Miyake S, Mizuno T, Tohyama M: Tumor necrosis factor-induces Bcl-2 and Bcl-x expression through NF-КB activation in primary hippocampus neurons. J Biol Chem 274:8531–8538, 1999
Chen C, Edelstein LC, Gelinas C: The Rel/ NF-КB family directly activates expression of the apoptotic inhibitor Bcl-x (L). Mol Cell Biol 20:2687–2695, 2000
Bentires-Alj M, Dejardin E, Viatour P, Van Lint C, Froesch B, Reed JC, Merville MP, Bours V: Inhibition of the NF-КB transcription factor increases Bax expression in cancer cell lines. Oncogene 20:2805–2813, 2001
Lin Y, Devine A, Rodriguez Y, Liu ZG: Cleavage of death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev 13:2514–2526, 1999
Duckett CS, Thompson CB: CD30-dependent degradation of TRAF-2: Implications for negative regulation of TRAF signaling and the control of cell survival. Genes Dev 11:2810–2821, 1997
Arch RH, Gedrich RW, Thompson CB: Translocation of TRAF proteins regulates apoptosis threshold of cells. Biochem Biophys Res Commun 272:936–945, 2000
Leo E, Deveraux QL, Buchholtz C, Welsh K, Matsuzawa S, Stennicke HR, Salvesen GS, Reed JC: TRAF1 is a substrate of caspases activated during TNF-α-induced apoptosis. J Biol Chem 276:8087–8093, 2001
Schwenzer R, Siewenzer R, Siemienski K, Liptay S, Schubert G, Peters N, Scheurich P, Schmid RM, Wajant H: The human TNFTRAF1 is up-regulated by cytokines of the TNF ligand family and modulates TNF-induced activation of NF-КB and c-jun N-terminal kinase. J Biol Chem 274:19368–19374, 1999
Tang G, Yang J, Minemoto Y, Li A: Blocking caspase-3-mediated proteolysis of IKKβ suppresses TNF-α-induced apoptosis. Mol Cell 8:1005–1016, 2001
Levkau B, Scatena M, Giachelli CM, Ross R, Raines EW: Apoptosis overrides survival signals through a caspase-mediated dominant-negative NF-КB loop. Nature Cell Biol 1:227–233, 1999
Clem RJ, Sheu TT, Richter BW, He WW, Thornberry NA, Duckett CS, Hardwick JM: cIAP-1 is cleaved by caspases to produce a proapoptotic C-terminal fragments. J Biol Chem 276:7602–7608, 2001
Deveraux QL, Leo E, Stennicke HR, Welsh K, Salvessen GS, Reed JC: Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J 18:5242–5251, 1999
Clem RJ, Cheng EH, Karp CL, Kirsch DG, Ueno K: Modulation of cell death by Bcl-XL through caspase interaction. Proc Natl Acad Sci USA 95:554–559, 1998
Fujita N, Nagahashi A, Nagashima K, Rokudai S, Tsuruo T: Acceleration of cell death after the cleavage of Bcl-XL protein by casopase-3 like proteases. Oncogene 17:1295–1304, 1998
Saville J, Fadok VA: Corpse clearance defines the meaning of cell death. Nature 407:784–788, 2000
Li H, Zhu H, Xu C-J, Yuan J: Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94(4):491–501, 1998
Luo X, Budihardjo I, Zou H, Slaughter C, Wang X: Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481–490, 1998
Perez D, White E: TNF-α signals apoptosis through a Biddependent conformational change in Bax that is inhibition by E1B 19K. Mol Cell 6:53–63, 2000
Higuchi M, Proske RJ, Yeh ETH: Inhibition of mitochondrial respiratory chain complex by TNF results in cytochrome c release, membrane permeability transition, and apoptosis. Oncogene 17:2515–2524, 1998
Pastorino JC, Chen ST, Tafani M, Snyder JW, Faber JL: The overexpression of Bax produces cell death upon induction of the mitochondrial permeability transition. J Biol Chem 273:7770–7775, 1998
Tafani M, Schneider TG, Pastorino JG, Farber JL: Cytochrome c-dependent activation of caspase-3 by tumor necrosis factor requires induction of mitochondrial permeability transition. Am J Pathol 156:2111–2121, 2000
Fernandez-Salas E, Sagar M, Cheng C, Yuspa SH, Weinberg WC: P53 and tumor necrosis factor-α regulate the expression of a mitochondrial channel protein. J Biol Chem 274:36488–36497, 1999
Goldstein J, Waterhouse N, Juin P, Evan G, Green D: The coordinate release of cytochrome “c” during apoptosis is rapid, complete and kinetically invariant. Nature Cell Biol 2:156–162, 2000
Marchetti P, Hirsch T, Zamzami N, Castedo M, Decaudin D, Susin SA, Masse B, Kroemer G: Mitochondrial permeability transition triggers lymphocyte apoptosis. J Immunol 157:4830–4836, 1996
Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD: The release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science 275:1132–1136, 1997
Yang J, Liu X, Bhalla K, Kim CN, Ibardo AM, Cai J, Peng TI, Jones DP Wang X: Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275:1129–1132, 1997
Gottlieb E, Vande Heiden MG, Thompson CB: Bcl-x (L) prevents the initial decrease in mitochondrial potential and subsequent reactive oxygen species production during tumor necrosis factor alpha-induced apoptosis. Mol Cell Biol 20:5680–5689, 2002
Macho A, Hirsch T, Marzo I, Marchetti P, Dallaporta B, Susin SA, Zamzami N, Kroemer G: Glutathione depletion is an early and calcium elevation is a late event of thymocyte apoptosis. J Immunol 158:4612–4619, 1997
Ghibelli L, Coppola S, Fanelli C, Rotilio G, Civitareale O, Scovassi AI, Ciriolo MR: Glutathione depletion causes cytochrome c release even in the absence of cell commitment to apoptosis. FASEB J 13(14):2031–2036, 1999
Ishii Y, Partridge CA, Del Vecchio PJ, Malik AB: Tumor necrosis factor-α-mediated decrease in glutathione increases the sensitivity of pulmonary vascular endothelial cells to H2O2. J Clin Invest 89:794–802, 1992
Hennet Y, Richter C, Peterhans E: Tumor necrosis factor-α induces superoxide anion production in mitochondria of L929 cells. Biochem J 289:587–592, 1993
Kimchi A: A cell death-promoting kinase. Nature Struct Biol 8:824–826, 2001
Raveh T, Droguett G, Horwitz MS, DePinho RA, Kimchi A: DAP kinase activates a p19ARF/p53-mediated apoptotic checkpoint to suppress oncogenic transformation. Nature Cell Biol 3:1–7, 2001
Cohen O, Inbal B, Kissil JL, Raveh T, Berissi H, Spivak-Kroizaman T, Feinstein E, Kimchi A: DAP-kinase participates in TNF-α-and Fas-induced apoptosis and its function requires the death domain. J Cell Biol 146:141–148, 1999
Woo RA, McLure KG, Lees-Miller SP, Rancourt DE, Lee PW: DNA-dependent protein kinase acts upstream of p53 in response to DNA damage. Nature 394:700–704, 1998
Tereshko V, Teplova M, Brunzelle J, Watterson MD, Egli M: Crystal structures of the catalytic domain of human protein kinase associated with apoptosis and tumor suppression. Nature Struc Biol 8:899–907, 2001
Gupta S: Tumor necrosis factor-α-induced apoptosis in T cell subsets from aged humans. Receptor expression and downstream signaling events. Exp Gerontol 37:293–299, 2002
Gupta S: A road to ruines: An insight into immunosenscence. Adv Cell Aging Gerontol (in press), 2002
Aggarwal S, Gollapudi S, Yel L, Gupta AS, Gupta S: TNF-α-induced apoptosis in neonatal lymphocytes: TNFRp55 expression and downstream pathways of apoptosis. Gene Immunity 1:271–279, 2000
Pahlvani M, Harris MD: The age-related changes in DNA binding activity of AP-1, NF-КB, and Oct-1 transcription factors in lymphocytes from rats. Age 19:45–54, 1996
Whisler RL, Beiqing L, Chen M: Age-related decreases in IL-2 production by human T cells are associated with impaired activation of nuclear transcriptional factors AP-1 and NF-AT. Cell Immunol 169:185–195, 1996
Trebilcock GU, Ponnappan U: Evidence for lowered induction of nuclear factor kappa B in activated human T lymphocytes during aging. Gerontology 42:137–146, 1996
Ponnappan U, Zhong M, Trebilcock GU: Decreased proteosomemediated degradation in T cells from the elderly: A role in immune senescence. Cell Immunol 192:167–174, 1999
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gupta, S. A Decision Between Life and Death During TNF-α-Induced Signaling. J Clin Immunol 22, 185–194 (2002). https://doi.org/10.1023/A:1016089607548
Issue Date:
DOI: https://doi.org/10.1023/A:1016089607548