Abstract
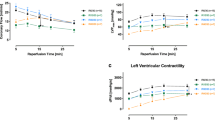
We investigated changes in cytoplasmic Ca2+ concentration ([Ca2+]i) and in left ventricular contractility during sustained ischemia and reperfusion in isolated beating rat hearts. Hearts from male Sprague-Dawley rats were perfused retrogradely and were loaded with 4 μM fura-2. Low-flow global ischemia was induced by reducing perfusion flow to 10% and by electric pacing. The hearts were exposed to ischemia for 10 min or 30 min and then reperfused. [Ca2+]i was measured by monitoring the ratio of 500 nm fluorescence excited at 340 and 380 nm while simultaneously measuring left ventricular pressure (LVP). To determine diastolic [Ca2+]i, background autofluorescence was subtracted. LVP rapidly decreased from 82.3 ± 8.2 to 17.1 ± 2.9 mmHg , whereas the amplitude of the Ca2+ transient did not change significantly during the first 1 min of ischemia. After 10 min of ischemia, the amplitude decreased to 60.8 ± 10.6% (p < 0.05) and diastolic [Ca2+]i increased by 26.3 ± 2.9% (p < 0.001) compared with the pre-ischemic value (n = 8). When the hearts were reperfused after 10 min of ischemia, the amplitude of the Ca2+ transient and LVP recovered to 79.0 ± 7.2% and 73.2 ± 7.5 mmHg, respectively. Whereas diastolic [Ca2+]i decreased to the pre-ischemic value. In the hearts exposed to 30 min of ischemia (n = 10), diastolic [Ca2+]i increased even further by 32.7 ± 5.3% at the end of ischemia and continued increasing during the 10 min of reperfusion by 42.6 ± 15.6%. Six of 10 hearts developed ventricular fibrillation (VF) and intracellular Ca2+ overload after reperfusion. Recovery of LVP after reperfusion was significantly smaller in the hearts exposed to 30 min of ischemia than in the hearts exposed to 10 min of ischemia (58.9 ± 11.7 vs. 97.2 ± 3.0% of pre-ischemic value, p < 0.05). Diastolic [Ca2+]i also increased under hypoxic conditions (N2 bubbling) in this model. These results suggest that increases in diastolic [Ca2+]i might play an important role in myocardial contractile dysfunction and viability in ischemia-reperfusion injury.
Similar content being viewed by others
References
Barry WH: Calcium and ischemic injury. Trends Cardiovasc Med 1: 162–166, 1991
Allshire AP, Piper HM, Cuthbertson KSR, Cobbold PH: Cytosolic free Ca2+ in single rat heart cells during anoxia and reoxygenation. Biochem J 244: 381–385, 1987
Haigney MCP, Miyata H, Lakatta EG, Stern MD, Silverman HS: Dependence of hypoxic cellular calcium loading on sodium-calcium exchange. Circ Res 71: 547–557, 1992
Seki S, MacLeod KT: Effects of anoxia on intracellular Ca2+ and contraction in isolated guinea pig cardiac myocytes. Am J Physiol 268: H1045–H1052, 1995
Ladilov Y, Haffner S, Balser-Schafer C, Maxeiner H, Piper HM: Cardioprotective effects of KB-R 7943: A novel inhibitor of the reverse mode of Na+/Ca2+ exchanger. Am J Physiol 276: H1868–H1876, 1999
Ishida H, Kohmoto O, Bridge JHB, Barry WH: Alterations in cation homeostasis in cultured chick ventricular cells during and after recovery from adenosine triphosphate depletion. J Clin Invest 81: 1173–1181, 1988
Murphy JG, Smith TW, Marsh JD: Calcium flux measurements during hypoxia in cultured heart cells. J Mol Cell Cardiol 19: 271–279, 1987
Nishioka K, Nakanishi T, Jarmakani JM: Effect of ischemia on calcium exchange in rabbit myocardium. Am J Physiol 247: H177–H184, 1984
Steenbergen C, Murphy E, Levy L, London R: Elevation in cytosolic free calcium concentration early in myocardial ischemia in perfused rat heart. Circ Res 60: 700–707, 1987
Lee HC, Mohabir R, Smith N, Franz MR, Clusin WT: Effect of ischemia on calcium dependent fluorescence transients in rabbit hearts containing indo 1: Correlation with monophasic action potentials and contraction. Circulation 78: 1047–1059, 1988
Kihara Y, Grossman W, Morgan JP: Direct measurement of changes in intracellular calcium transients during hypoxia, ischemia, and reperfusion of the intact mammalian heart. Circ Res 65: 1029–1044, 1989
Lorell BH, Apstein CS, Cunningham MJ, Schoen FJ, Weinberg EO, Peeters GA, Barry WH: Contribution of endothelial cells to calcium-dependent fluorescence transients in rabbit hearts loaded with indo 1. Circ Res 67: 415–425, 1990
Mohabir R, Lee H-C, Kurz RW, Clusin WT: Effects of ischemia and hypercarbic acidosis on myocyte calcium transients, contraction, and pHi in perfused rabbit hearts. Circ Res 69: 1525–1537, 1991
Kato Y, Otani H, Tanaka K, Saito Y, Fukunaka M, Imamura H: Effect of cardioplegic preservation on intracellular calcium transients. Ann Thorac Surg 52: 979–986, 1991
Camacho SA, Figueredo VM, Brandes R, Weiner MW: Ca2+-dependent fluorescence transients and phosphate metabolism during low-flow ischemia in rat hearts. Am J Physiol 265: H114–H122, 1993
Liu X, Engelman RM, Rousou JA, Flack JE, Deaton DW, Das DK: Normothermic cardioplegia prevents intracellular calcium accumulation during cardioplegic arrest and reperfusion. Circulation 90: II-316–II-320, 1994
Field ML, Azzawi A, Styles P, Henderson C, Seymour A-ML, Radda GK: Intracellular Ca2+ transients in isolated perfused rat heart: Measurement using the fluorescent indicator Fura-2/AM. Cell Calcium 16: 87–100, 1994
Brooks WW, Conrad CH, Morgan JP: Reperfusion induced arrhythmias following ischaemia in intact rat heart: Role of intracellular calcium. Cardiovasc Res 29: 536–542, 1995
Taniguchi M, Okumura H, Anzawa R, Seki S, Taniguchi I, Izumi T, Date T, Miyazaki H, Mochizuki S: Ischemic preconditioning attenuates decrease in intracellular pH and accumulation of intracellular Ca2+ and Na+. Circulation 94: (abstr) I-363, 1996
Andre Ng G, Cobbe SM, Smith GL: Non-uniform prolongation of intracellular Ca2+ transients recorded from the epicardial surface of isolated hearts from rabbits with heart failure. Cardiovasc Res 37: 489–502, 1998
Hotta Y, Fujita M, Nakagawa J, Ando H, Takeya K, Ishikawa N: Contribution of cytosolic ionic and energetic milieu change to ischemia-and reperfusion-induced injury in guinea pig heart: Fluorometry and nuclear magnetic resonance studies. J Cardiovasc Pharmacol 31: 146–156, 1998
Nishida M, Borzak S, Kraemer B, Nava JP, Kelly RA, Smith TW, Marsh JD: Role of cation gradients in hypercontracture of myocytes during simulated ischemia and reperfusion. Am J Physiol 264: H1896–H1906, 1993
Kaplan P, Hendrikx M, Mattheussen M, Mubagwa K, Flameng W: Effect of ischemia and reperfusion on sarcoplasmic reticulum calcium uptake. Circ Res 71: 1123–1130, 1992
Chen W, Steenbergen C, Levy L, Vance J, London R, Murphy E: Measurement of free Ca2+ in sarcoplasmic reticulum in perfused rabbit heart loaded with 1,2–bis (2 Amino-5 (2–amino-5,6–difluorophenoxy) ethane-N,N,N′,N′-tetraacetic acid by 19F NMR. J Biol Chem 271: 7398–7403, 1996
Callewaert G, Cleemann L, Morad M: Caffeine-induced Ca2+ release activates Ca2+ extrusion via Na+-Ca2+ exchanger in cardiac myocytes. Am J Physiol 257: C147–C152, 1982
Mochizuki S, MacLeod KT: The effects of hypoxia and metabolic inhibition on increases in intracellular Ca2+ concentration induced by Na+/Ca2+ exchange in isolated guinea-pig cardiac myocytes. J Mol Cell Cardiol 29: 2979–2987, 1997
Tani M, Neely JR: Role of intracellular Na+ in Ca2+ overload and depressed recovery of ventricular function of reperfused ischemic rat hearts. Possible involvement of H+-Na+ exchange and Na+-Ca2+ exchange. Circ Res 65: 1045–1056, 1989
Murphy E, Perlman M, London RE, Steenbergen C: Amiloride delays the ischemia induced rise in cytosolic free calcium. Circ Res 68: 1250–1258, 1991
Koomen JM, Schevers JAM, Noordhoek J: Myocardial recovery from global ischemia and reperfusion: Effects of pre-and/or post-ischemic reperfusion with low-Ca2+. J Mol Cell Cardiol 15: 383–392, 1983
Bourdillon PDV, Poole-Wilson PA: Effects of ischemia and reperfusion on calcium exchange and mechanical function in isolated rabbit myocardium. Cardiovasc Res 15: 121–130, 1981
Zaugg CE, Wu ST, Barbosa V, Buser PT, Wikman-Coffelt J, Parmley WW, Lee RJ: Ventricular fibrillation-induced intracellular Ca2+ overload causes failed electrical defibrillation and post-shock reinhibition of fibrillation. J Mol Cell Cardiol 30: 2183–2192, 1998
Maddaford TG, Pierce GN: Myocardial dysfuncton is associated with activation of Na+/H+ exchange immediately during reperfusion. Am J Physiol 273: H2232–H2239, 1997
Cross H, Lu L, Steenbergen C, Phillipson K, Murphy E: Overexpression of the cardiac Na+/Ca2+ exchanger increases susceptibility to ischemia/reperfusion injury in male, but not female, transgenic mice. Circ Res 83: 1215–1223, 1998
Miyata H, Lakatta EG, Stern MD, Silverman HS: Relation of mitochondrial and cytosolic free calcium to cardiac myocyte recovery after exposure to anoxia. Circ Res 71: 605–613, 1992
Maddaford TG, Hurtado C, Sobrattee S, Czubryt MP, Pierce GN: A model of low-flow ischemia and reperfusion in single, beating adult cardiomyocytes. Am J Physiol 277: H788–H798, 1999.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seki, S., Horikoshi, K., Takeda, H. et al. Effects of sustained low-flow ischemia and reperfusion on Ca2+ transients and contractility in perfused rat hearts. Mol Cell Biochem 216, 111–119 (2001). https://doi.org/10.1023/A:1011067529272
Issue Date:
DOI: https://doi.org/10.1023/A:1011067529272