Abstract
Climate change has posed a significant threat to agricultural productivity in recent years. Due to their sessile nature, plants are vulnerable to various biotic and abiotic stresses. Heat stress has emerged as the most significant abiotic stress in recent years, as global temperatures rise daily. Heat stress inhibits photosynthesis, plant growth, pollen production, and reproduction. The plant's photosynthetic efficiency is mainly reduced by the overproduction of reactive oxygen species, denaturation of heat shock proteins, and alterations in several enzyme activities. Phytohormones have been shown to provide a comprehensive mechanism for stress resistance at numerous biochemical, molecular, and physiological levels, indicating that they play an essential role in plant growth and development. The various stressors primarily cause nutritional deficits and limit plant nutrient absorption. Phytohormones can enhance heat stress tolerance by boosting seed germination, antioxidant enzymes, leaf photosynthesis, seedling growth, and root growth while decreasing electrolyte leakage, reactive oxygen species, and malonaldehyde. At the same time, plant exogenous nutrients such as Ca, K, Mg, and N also engage in ROS scavenging activities by boosting antioxidant qualities, increasing photosynthetic potential, and decreasing cell membrane leakage by resynthesizing chlorophyll pigments. To cope with heat stress, plants have evolved adaptive systems. Plant defense responses to various abiotic challenges, including heat, are regulated by the classical plant hormones, which include auxin, cytokinin, abscisic acid, brassinosteroids, jasmonate, ethylene, and salicylic acid. These hormones integrate environmental stimuli and endogenous signals. Plants that receive exogenous administration of those hormones before or after heat stress are more thermotolerant. This review focused on the physiological, molecular and biochemical aspects that plants experience due to heat stress and the role of exogenous phytohormones and plant nutrients in mitigating heat stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Global temperature increases significantly threaten food security, impacting crop productivity and the reproductive phase of plants, which is vital for managing future agricultural productivity and addressing the burgeoning human population [1]. Heat stress (HS) encompasses a range of biochemical and physiological disruptions triggered by temperatures exceeding the optimal range for plant development. Several factors can significantly hamper plant growth, development, and production. These include reduced photosynthetic efficiency, protein denaturation, instability of cellular membranes, and an increase in the creation of reactive oxygen species (ROS) [2]. HS directly curtails photosynthesis, elevates respiration, denatures proteins, induces oxidative stress, disrupts hormone balance, triggers water stress, and compromises reproductive system development. The potential global losses from heat and drought catastrophes between 1961 and 2014 were projected to be 0.5% of oil seed crops, 1.4% of grain production, 0.09% of vegetables, 0.2% of fruits, and 0.6% of pulses [3].
The three most important development phases (booting, anthesis, and grain filling) in T. aestivum are impacted by HS [4]. When the daily temperature exceeded the threshold level of 30 °C, Zea may’s kernel production decreased by 1% per day [5]. Compared to ongoing moderate stress, a brief period of high temperatures significantly affects grain growth. In Oryza sativa, higher nighttime temperatures exhibited more detrimental effects than daytime temperatures [6]. Various factors, including the type of plant, growth stage, length, and intensity of heat exposure, influence the severity of the impacts of HS on plants. Understanding and mitigating HS is essential for plant survival and agricultural output.
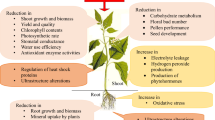
The global food deficit persists, with declining cereal crop yield rates due to high-temperature stress affecting reproductive development and pollen infertility. Plants create defense mechanisms to lessen stress by manufacturing phytohormones, including JA (jasmonic acid), ethylene, polyamines, IAA (indole acetic acid), melatonin, BRs (brassinosteroid), abscisic acid, SA (salicylic acid), PGPR-mediated phytohormones, and GA (gibberellic acid) [7]. Furthermore, novel plant growth regulators (strigolactones) have been identified [8]. To protect cells from heat-induced damage, salicylic acid (SA) increases the synthesis of antioxidants and heat shock proteins [9]. Despite being linked to the ripening and senescence of fruit, ethylene also influences the expression of genes related to stress responses, which helps plants tolerate HS. By boosting the pace of photosynthetic reaction and upregulating heat shock protein (HSP) expression, BRs improve plant thermotolerance. Under temperature stress conditions, the BR signaling pathway triggers the production of PIFs and orchestrates changes in plant architecture. ROS buildup is inevitable due to HS. Therefore, to increase heat tolerance, most hormones regulate plant ROS homeostasis by scavenging ROS and producing antioxidants [10]. Applying phytohormones externally significantly reduced heat-induced damage and increased plant tolerance to heat, suggesting that phytohormones have a role in the plant's reaction to HS. Phytohormones' signaling mechanisms and biosynthesis have been well-explained in recent studies, primarily about the model plant Arabidopsis thaliana [10, 11]. In response to changing temperatures, there may be chances to develop thermotolerant varieties and cultivate agriculturally significant crop cultivars by studying the molecular mechanisms underpinning plant hormone-mediated heat response [12]. To synchronize plant development and stress defense, auxin and the auxin pathway control plant thermomorphogenesis in response to HS. By minimizing oxidative damage and preserving photosynthesis, ABA and SA help plants recover from the damaging effects of HS [10]. To control a plant's defensive response to various abiotic challenges, including heat, the classical plant hormones auxin, JA, SA, cytokinin, BRs, ABA, and ethylene combine environmental cues and endogenous signals (Fig. 1). Plants become more thermotolerant when those hormones are applied exogenously before or after HS. Such phytohormones are still in their infancy when providing plant adaptability to dynamic climatic changes [13].
On the other hand, Plant nutrients (Nitrogen, Potassium, Calcium, Magnesium, Boron, Zinc, and Selenium) are necessary to regulate osmotic pressure, enzyme activation, and energy production and form the structural components of nucleic acids. These processes help maintain plant architecture, support essential physiological functions, and enhance stress tolerance (Fig. 1). Nitric oxide (NO), a highly reactive free radical that permeates membranes [14], is a form of nitrogen that has a wide range of regulatory roles in numerous physiological processes, including ethylene emission, stomatal closure, heat stress, drought stress, salinity, and cell senescence. NO also acts as a signaling molecule that mediates responses to abiotic and biotic stresses, including these [15]. To lessen HS's consequences, potassium is essential for osmoregulation, enzyme activity, and cellular ion balance maintenance [16].
Previous studies only discuss one topic, either phytohormones or plant nutrients. The current review addressed heat stress's impacts on plants’ physiological, molecular and biochemical aspects. It also discussed the roles of certain exogenous nutrients (Nitrogen, Potassium, Calcium, Magnesium, Boron, Zinc, and Selenium) and phytohormones in reducing the adverse effects of HS on plants. This review addresses some research gaps and future work from which researchers can learn a new way of research. Due to climate change, HS is now one of the major issues affecting crop production. To increase the sustainability of plants, it is necessary to have a proper and deep knowledge of the mechanism and use of phytohormones and plant nutrients.
2 Methods and framework
The impact of plant nutrients and exogenous phytohormones on heat-stressed plants has been investigated by browsing many scholarly databases. Google Scholar, Springer Link, Web of Science, Wiley Online Library and Mendeley were used to search for scientific literature. We used the keywords ‘Phytohormones’, ‘Plant nutrients’, ‘Heat stress’, ‘Mitigation of heat stress’, ‘Heat stress adoption’, ‘Effect of heat stress on plant’, ‘Role of phytohormone in abiotic stress tolerance’, ‘Role of plant nutrients in abiotic stress tolerance’ for finding related papers. We picked a few research and review publications according to predetermined standards. The following criteria were used for the selection: (i) the study includes both phytohormones and heat stress; (ii) the study includes both plant nutrients and heat stress; (iii) the study includes the effects on plants in heat stress; and (iv) the study includes present strategies of mitigating heat stress using phytohormones and plant nutrients.
The review is divided into six major stages (Fig. 2). A detailed explanation of how HS affects plant functions in the first stages. The second stage involves the application of some selected phytohormones (Gamma-aminobutyric acid (GABA), Abscisic acid, Cytokinins, Jasmonates, Melatonin, Salicylic Acid, Strigolactones, Gibberellins, Brassinosteroids, Auxin, and Ethylene) to lessen HS. The third section describes how exogenous nutrients (some selected), such as zinc, boron, magnesium, calcium, potassium, selenium, and potassium, might lessen HS.
The fourth stage highlights the significance of external applications of phytohormones and plant nutrients for sustainable agriculture. The fifth stage outlines the general issues we faced when constructing our review. Lastly, the research gap and upcoming works are included in the final step. This point could assist readers or researchers in finding out new areas of research.
3 Plant functions affected by heat stress
3.1 Photosynthesis
In the earliest stages of photosynthesis, chlorophyll, the principal photosynthetic pigment, could absorb light energy with transmit electrons. Chl-degrading peroxidase and Chlorophyllase dramatically increase activity when exposed to HS. The action of Chl-degrading peroxidase and chlorophyllase both significantly increases during HS. Consequently, there is a notable reduction in Chl levels. Plant photosynthesis is impacted when chlorophyll absorbs too much light energy. The balance between Chl production and breakdown is crucial for sustaining the photosynthetic resources and promoting photosynthetic efficiency, affecting crop development and production [17]. HS changes leaves' morphology, architecture, physiology, and photosynthetic capability, the central organ used for photosynthesis and transpiration. This results in wilting and other modifications.
3.1.1 Leaf anatomical structure
The equilibrium of energy between the interior of the leaf and its surroundings is significantly influenced by the temperature of the leaf. Several investigations were conducted to examine the winter wheat's leaf area index (LAI) grown in the North China Plains under high temperatures (HS) of > 34 °C. The results indicated that HS sped up the senescence of the leaf and reduced LAI, as shown in maize [18], wheat [19] and tomatoes [20]. These findings harmed photosynthetic performance.
Upon analyzing the anatomy of the leaf of multiple cultivars of Rhododendron × hybridum, Glenn Dale Azalea, cultivated at 38/30◦C (HS) when control is 25/17◦C, the researchers discovered that HS noticeably increased the length of stomata (54.3%) in cultivar 'Liu Qiu Hong', but only by 10.8% in cultivar 'Lan Yin'. Regarding palisade tissues, cuticle length, vascular components, and stomata (HS reduced around 50% of open stomata), Lan Yin even beat Liu Qiu Hong [21].
The percentage of open stomata and the reduced size slow down Lan Yin's transpiration water losses and photosynthetic efficiency, indicating that stomatal variables are critical for HS tolerance. Once more, rice under HS (38/28 ◦C) had lower stomatal density than control (30/28 ◦C) plants.
Regardless of stress treatments, the Oryza sativa L. javanica (javanica rice) varieties had more minor vein densities and larger veins. In contrast, the Oryza sativa L. indica (indica rice) types had more vein and stomata densities [22].
According to a study, petiole lengths in two genotypes of Brassica campestris L. (field mustard) -WS-1 (heat-tolerant genotype) and WS-6 (heat-susceptible genotype)—were found to be reduced by 19.4% and 16.88%, respectively, after exposure to higher temperatures for seven days. Palisade and spongy mesophyll cells were arranged loosely and disorderly in both genotypes; under HS, these models thinned more in WS-6 than in WS-1. The amount of the palisade mesophyll and the top epidermis in WS-6 was seen to be lowered by 10.13% and 25.27%, respectively, while the volume of the spongy mesophyll, leaf lamina and palisade mesophyll in WS-1 increase by 42.06%, 22.31% 14.83%, and 24.66%, accordingly due to HS [23].
3.1.2 Photosynthetic proteins and pigments
Light energy is converted into chemical energy during photosynthesis, and photosynthetic pigments are essential. Photosynthetic proteins significantly regulate plant health and photosynthetic electron transport. Plant chlorophyll declines under HS, resulting in chlorosis or senescence of the leaf [24]. Wheat was shown to have lower levels of total chlorophyll (44.12%), chlorophyll b (56.52%), chlorophyll a (38.05%), and carotenoid (48.27%) during the heading stage when exposed to HS (37 ± 2 °C) [25].
It follows that HS impacts all photosynthetic pigments. Photosynthetic pigment proteins underwent a decrease under HS, as documented in many plants such as alfalfa [26], tomato [27], wheat [28] and quinoa [29]. However, it was noted that rice grown under HS had higher photosynthetic contents [30].
3.1.3 Ultrastructure of chloroplast
Particularly in chloroplasts, HS causes distortions in the ultrastructure of cellular organelles. It is reported that utilizing model plants such as green foxtail, HS (40 °C) enhanced the grana size in chloroplasts of mesophyll but had minimal impact on the buildup of starch or the density of chloroplasts [31].
Furthermore, in mesophyll chloroplasts and bundle sheath, HS strongly stimulated the production of plastoglobuli, which was significantly linked with leakiness of the thylakoid membrane and damage to the ultrastructure of the chloroplasts. A study found that in herbaceous Paeonia lactiflora Pall. (peony), Heat exposure (40/35 °C) for 14 days caused damage to segregated plastid walls, residual amyloplasts, and thylakoid membranes [32].
Moreover, HS harmed the chloroplasts' inner membrane system, causing osmiophilic particles to agglomerate. This suggests that various plant species have distinct responses to the chloroplast ultrastructure. Furthermore, compared to control plants, HS (38/30◦C) significantly altered the chloroplast and thylakoid ultrastructure [33]. They were increasing the number of plastoglobuli, reducing the size of starch granules, reducing grana stacking, disorganizing thylakoids, distorting inter-lamellar granular and stroma systems, and excessively swelling the membrane around the outer chloroplast. HS damages chloroplast ultrastructure and reduces photosynthetic effectiveness [34, 35]. An overview of how HS affects some plants' photosynthetic systems is provided in Table 1.
3.2 Production of ROS
ROS is a typical consequence of plant metabolism, mainly produced within chloroplasts and mitochondria. Under heat exposure, they showed a notable uptick in activities [41]. To accurately grasp ROS's involvement and function in plant cells' growth and development during HS, one needs to be conscious of the peculiarities of the cells in plant tissue being examined and approach the research cautiously. A study suggested that applying HS to winter wheat increased the activity of mitochondrial ROS generation [42].
Based on the tissue and organ type, ROS buildup during HS serves a dual role in tip growth and cell proliferation. The ROS threshold in cells is controlled by antioxidants like ascorbic acid (AsA), glutathione (GSH) and flavonols [43,44,45]. High temperature raises the quantity of flavonoids in polarised tomato microspores concerning modifications to the secondary metabolite [46]. ROS detoxification mainly depends on flavonoids [47]. In late pollen developmental stages under HS, conjugated polyamine abundance is 37% lower than in polarised microspores [46]. These polyamines, which aid in the detoxification of ROS, increase the level of antioxidant enzyme activity [48].
As a byproduct of aerobic metabolism, the large number of mitochondria caused by HS leads to a sharp increase in ROS formation [49], and these high levels result in cell death and oxidative damage [50]. ROS signalling is crucial for the tapetum's embryonic programmed cell death (PCD) in monocot species like rice (Oryza sativa) and dicot species including tomato, Arabidopsis, and tobacco [51].
ROS is essential for controlling the formation of pollen tubes, root hair cells, pathogen defense, and immunological response to abiotic stressors such as HS [52, 53]. The mismatch between ROS quenching enzymes and ROS level brought on by ROS accumulation in anthers under HS resulted in degeneration of the tapetal cell layer and early PCD [54]. HS increases the generation of ROS and produces cell elongation, which reduces fertilization efficiency. However, ROS is becoming increasingly recognized as an essential signaling molecule that aids in adapting plants to abiotic stimuli such as HS.
3.3 Plant growth and development
HS adversely affects plant growth and development at all stages, from germination to harvesting. It is defined as a temperature rise of 10–15 °C over the surrounding air. It presents several physiological and morphological difficulties for plants, including decreased shoot and root growth, root count, diameter and height, as well as yellowing, curling, and leaf withering [55, 56]. Furthermore, this condition has been shown to significantly impact plant development and production due to excessive radiation and high temperatures, which function as limiting factors, causing crop harm that encompasses foliage, appendages, and stalks. Moreover, high temperatures can decrease weight during shoot development, leading to early plant mortality [57]. Additionally, Temperature variations can impact various plant processes, including root development, nutrient uptake concentration, nutrient-assimilation proteins, and root uptake velocity [58]. Temperature variations have the potential to significantly affect a plant's photosynthetic activity, which could lead to a notable reduction in plant growth and production [56, 59]. The physio-morphological symptoms include sunburns on leaves and sensitive twigs and branches, senescence and abscission of the leaf, decreased growth of shoot and root, and discoloration and damage to fruits. It can also modify plant hormone balance, stability, biosynthesis, and compartmentalization, consequently altering plant growth and development [60]. Physiologically, it damages membrane lipids, carbon and nitrogen metabolism, light-driven carbon fixation, yield production, and grain quality, all impacting plant growth and development [61]. Not only does it denature proteins and enzymes, lowering nutrient and water absorption crucial for plant development, but it also dehydrates, hindering growth by significantly dropping water potential and relative water content. Plant growth is significantly hampered by temperatures of 45 °C or higher, disrupting cellular balance, stunting growth, and damaging proteins, rendering cells defenseless [62, 63]. When rice is exposed to temperatures above 40 °C for a long time, its development is inhibited due to a reduced proline concentration [30]. The growth of wheat plants is adversely affected, leading to increased oxidative stress. According to research, under normal conditions, wheat plants reach a height of 66.4 to 97.3 cm, whereas under it, this range decreases to 55.7 to 82.3 cm [64, 65]. Extreme heat damages sugarcane leaf tips and margins, drying them out and causing necrotic lesions due to cell death. It also disrupts sugar metabolism, reducing grain yield, pollen sterility, impeding growth, and failed fertilization [66]. It decreased water status and cell size, which in turn caused growth loss in pearl millet and maize (Pennisetum glaucum L.) [59, 62]. Furthermore, it decreased crop growth rate and dry matter output in various plants, particularly sugarcane (Saccharum officinarum L.). Moreover, extreme and mild HS causes the plant to lose its leaves, abort flowers and fruits, and die [3, 62]. The Common bean (Phaseolus vulgaris) severely impairs morpho-physiological traits, including growth and development [59]. Orchard grass and other perennial grass species experienced reduced growth due to it, affecting both warm-season species like Andropogon gerardi and Solidago canadensis, with the latter more significantly impacted. This condition also notably altered hormone levels in perennial ryegrass, affecting auxin, ABA, cytokinin and reducing GA3 concentration [60]. Apart from the adverse effects, it has some positive sides too. Heat levels over the ideal threshold boosted crop development by speeding up biological activities, which shortened the growing season [3]. Addressing the impact of this condition on plant growth and development is essential, as it presents a significant challenge with wide-ranging physiological and morphological implications.
3.4 Pollens and pollination in crops
An important first step in creating breeding plans to produce high and consistent yields in crop plants will be recognizing how HS affects pollen. Compared to the vegetative phase of plant growth, the reproductive phase is more susceptible to high temperatures. So, more attention needs to be paid to this phase of the study to understand plant resistance to HS better. Environmental factors impact all stages of reproductive development, including ovule and pollen formation, pollen tube expansion, male–female communication, fertilization, and embryo development [67].
Heat-stressed plants exhibit inadequate pollination, which results in spikelet damage because of pollen grain deposition is inadequate and inadequate fertilization from weak pollen germination on the stigma. HS has been discovered to negatively affect rice spikelet fertility throughout the flowering stage [68]. Elevated temperatures frequently cause adverse effects on the sexual reproduction of plants, resulting in the infertility of numerous species. Development of embryo and seed, progamic phase, and gametophyte development are the three stages of sexual reproduction in angiosperms [69].
Pollens are more vulnerable to HS during development because HS changes the form and quantity of pollen, the cell wall's structure, and particularly pollen's metabolism [70]. However, when using the high-temperature modes, sensitivity to HS differs from species to species [71]. Pollen is the weakest link when HS is used before pollination, independently to male and female gametes, according to a study that used crosses of Solanum lycopersicum (tomato) and Brassica napus plants with female and male reproductive organs separately under various high-temperature stresses [72]. Once more, HS application during this window of development can seriously disrupt reproductive development and cause pollen abortion, which impairs fertilization. In an experiment, when Arabidopsis and Rosa spp. (rose) plants are exposed to mild HS, 48 h at 36 °C, restored meiotic triads and dyads, carrying unreduced, diploid male gametes in the place of the usual haploid ones [73, 74]. Furthermore, influencing meiosis, HS modifies spindle orientation and cytoskeletal dynamics in tobacco (Nicotiana tabacum) and Arabidopsis [71, 75].
HS causes premature degeneracy of the tapetum. It is a layer of nutrient-rich cells that nourish pollen grains as they mature. As soon as pollen gets to the stigma, the progamic phase begins. To achieve twofold fertilisation, male gametes are transported to the embryo sac via highly specialized structures called pollen tubes [76]. In tobacco pollen tubes, HS alters isoform distribution and subunits' composition cytoskeletal, which affects the callose synthases and distribution of cellulose. Two enzymes participate in the manufacture of cell walls and the collection of secretory vesicles.
Table 2 summarizes several published studies to help understand how HS affects male gametophytes. Additional effects of high-temperature exposure on the organic phase include reduced ovule viability, changes in stigma and style placement, and decreased stigma responsiveness, all of which lead to insufficient fertilization.
3.5 Grain yield production
A considerate negative correlation between seasonal high temperatures and crop yield is associated with HS [83, 84]. One day of HS can reduce grain production significantly [85]. In maize, for every increasing degree in temperature, production will drop by 7.4% [86, 87]. Wheat under HS reduces translocation to developing grains, lowering grain production and grain size [88, 89].
Delays in seeding resulted in terminal heat stress, which had a detrimental effect on thousand kernel weights, biological yield, and grain production [90]. During the reproductive stage, heat severely stresses wheat plants, which lowers spike length, the number of grains, plant height, and total grain yield [91]. Excess heat diminishes the pre-flowering integration supply at the panicle initiation stage and inhibits sink capacity by decreasing grain size [92]. Before anthesis, temperature above 31 °C causes pollen sterility, which means pollen cannot fertilize ovules and generate viable seeds, generating pre-anthesis disturbances that ultimately impact yield components and total production [93]. HS at anthesis primarily decreases grains per plant, resulting in low yield and post-anthesis. It mainly affects grain yield by reducing thousand kernel weight and grain size [94]. In wheat, higher temperatures reduce the rate of gain filling by shortening the days needed for anthesis. Also, high temperatures diminish pollen fertility and the formation of pollen tubes and impede the expansion of ovary and pollen growth, reducing peer fertilization and, eventually, seed setting [95] (Table 3). There was a significant decrease in wheat grain yield, with a reduction of 57.3%, caused by intense HS during the grain filling period [89, 96] and short-term HS reduced 23% grain yield [97].
4 Phytohormones effect on mitigation of heat stress
When plants are exposed to HS, phytohormones are essential for their survival and growth. Plants can adapt to and lessen the negative impacts of high temperatures by regulating various physiological and molecular responses. The primary phytohormones involved in HS reactions are strigolactones, gibberellins, cytokinins, ethylene, jasmonates, melatonin, salicylic acid (SA), abscisic acid (ABA), auxin Gamma-aminobutyric acid (GABA) and brassinosteroids (BRs). These hormones' distinct modes of action influence plants' ability to withstand HS. Their actions in mitigating HS are shown in Fig. 3.
4.1 Abscisic acid
Abscisic acid is an important plant hormone that controls stress responses. ABA, often known as the "stress hormone," is released into the environment when a plant is exposed to harsh conditions like drought, excessive salt, or extremely high temperatures [124]. This increase in ABA functions as a signal, activating a cascade of cellular reactions that improve the plant's resistance to various stimuli. The ABA uses stomatal closure as one of its primary mechanisms. In drought, ABA signaling encourages the closing of tiny pores on the leaf surface, known as stomata. When cells are exposed to HS, the production and distribution of ABA cause stomatal closure and a reduction in transpiration rate, which restricts cell growth [125]. This closure limits the amount of water lost through transpiration, an essential adaptation for protecting valuable water supplies during dry spells [126]. Additionally, in unfavorable circumstances, ABA can cause seed dormancy. ABA delays germination to promote good seedling establishment, ensuring that seeds remain viable until a more favorable environment with enough water and nutrients becomes available [127]. Additionally, by enhancing antioxidant capacity, controlling osmotic pressure, and encouraging photosynthesis, ABA might significantly improve tomato seedlings' ability to withstand salt [128]. A plant's ability to adjust to external stressors, control water levels in the plant body through stomatal opening and shutting, and develop seeds and dormancy are all impacted by ABA. When there is a water shortage, ABA is biosynthesized in the roots and transported to the leaves through the xylem, raising the concentration of ABA in the leaves. Additionally, Plant stress tolerance is enhanced by modulating antioxidant enzyme activities, stabilizing cellular structures, and regulating gene expression associated with stress responses, all of which act as molecular chaperones to protect cellular proteins from denaturation and aggregation under high temperatures [129, 130]. These transcription factors bind to ABA-responsive elements (ABREs) in target genes' promoters, regulating their expression and improving stress tolerance. Upregulated genes frequently encode protective proteins such as heat shock proteins (HSPs) and enzymes that create osmoprotectants and antioxidants (Fig. 3). ABA plays a vital role in maintaining cellular homeostasis and shielding plants from the adverse effects of HS via this regulatory network, contributing to overall plant resilience and survival in harsh environmental circumstances [131]. This ABA-mediated response is critical for maintaining cellular integrity and sustaining plant survival at high temperatures [132]. To sum up, ABA functions as a complex signaling molecule that coordinates various physiological reactions that enable plants to withstand various environmental stressors. ABA helps plants preserve water, shield their cells, and delay development until more favorable conditions arise, improving their survival and reproductive success. It also encourages stomatal closure, seed dormancy, and antioxidant defenses.
ABA promotes stomatal closure in drought-prone areas, which is beneficial since it reduces transpiration-related water loss. Furthermore, ABA is necessary to postpone germination until an environment more favorable for seedling growth is present since it induces seed dormancy in response to unfavorable conditions. These findings highlight the function of ABA in enhancing plant tolerance to external stresses.
4.2 Cytokinins
Cytokinins are one of the ubiquitous phytohormones that participate in morphogenesis, development, and many other processes involved in plant physiology [133]. These are some of the primary plant hormones that regulate various plant growth and development processes. Several studies have proven that cytokinins play a vital role in heat-stress mitigation in plants [134]. Cytokinins not only elevate the activities of enzymes such as Ascorbate Peroxidase (APX), Superoxide Dismutase (SOD) and Glycogen Phosphorylase (GP), but they also upregulate many genes that are responsible for physiological processes like photosynthesis and carbohydrate metabolism under heat-induced stress conditions [135]. Besides, cytokinins promote transpiration, which aids in heat dissipation and encourages the production of heat shock proteins, which protect plants from heat-stress conditions with the help of the proteins [136].
Transgenic plants containing high cytokinin levels show improved heat resistance, but plants with low cytokinin levels are more vulnerable to HS [137]. Overexpression of genes that regulate cytokinin biosynthesis, like IPT, elevates endogenous cytokinin levels and enhances heat-stress tolerance capacity in grasses [138]. Furthermore, ectopic expression of isopentenyl transferase (ipt) to increase heat-stress tolerance has been experimented with in Agrobacterium tumefaciens [139]. Several studies in passion fruit, rice (Oryza sativa) and Arabidopsis have shown that if cytokinin is applied externally, it reduces the negative impacts of HS [140]. High temperatures cause Passiflora edulis flowers to die, but cytokinin treatment improves heat tolerance and reduces the abortion of flowers [137]. Moreover, applying cytokinins to wheat plants' post-anthesis period increased grain yields and heat tolerance in the cultivar "Wennong6" [141]. In bentgrass, cytokinin application repressed leaf senescence caused by HS, resulting in higher turf quality, a rise in chlorophyll content, and a decrease in electrolyte leakage [142].
The rapid heat-stress response involves a complex signaling network mediated by phytohormones such as cytokinin, which activate HS factors and related proteins [143]. In Arabidopsis, thermal stress induces the expression of HS-related genes and proteins. Exposing non-stressed organs to heat reduces abscisic acid and active cytokinin levels but increases cytokinin in leaves, stimulating components of the cytokinin signaling pathway and enhancing transpiration and photosynthesis [135]. Though the roles of cytokinins in developmental processes are well-characterized, their effects on abiotic stress tolerance, such as HS, remain inconsistent due to the complex relationship between cytokinin and stress signaling [144]. However, inhibitors of cytokinin oxidase or dehydrogenase, enzymes that degrade cytokinins, have been shown to improve HS mitigation [145]. By inhibiting these enzymes, cytokinin levels are maintained, enhancing the plant's ability to cope with HS.
The potential of cytokinins to mitigate HS through genetic manipulation, external application, and inhibition of degradation enzymes provides promising aspects for enhancing plants' ability to thrive in rising temperatures. Future research should focus on elucidating the complex signaling networks, cytokinins, and other phytohormone interactions to develop more effective strategies for heat-stress-tolerant plants. More research is necessary to fully understand the intricate signaling network involving cytokinin and other hormones like abscisic acid. Targeted manipulation requires knowledge of the distinct cytokinin types and their associated signaling pathways in various plant species. It is necessary to look at any potential adverse effects of prolonged cytokinin manipulation, such as disturbances to normal plant development. Optimizing timing and cytokinin delivery methods (spraying/root drenching) for particular crops and stress intensities is necessary to maximize advantages. Furthermore, it would be beneficial to investigate how cytokinin interacts with other stress signaling pathways and functions in mitigating other abiotic stresses. In general, this analysis provides a basis for further investigation to fully realize the promise of cytokinins in creating heat-tolerant plants and creating strategies to reduce stress in an increasingly heated global environment.
4.3 Jasmonates
Plant signaling molecules significantly impact how plants react to environmental stressors. They also have various functions in the growth and maturation of plants [146, 147]. Jasmonates are one of the plant signaling molecules. There is clear evidence that jasmonic acid (JA) plays a significant role in controlling procedures such as photosynthesis, chlorophyll breakdown, root elongation, stomatal growth, and leaf senescence. Jasmonates (JAs) are a class of multifunctional chemicals that include methyl jasmonate (MeJA) and jasmonic acid (JA) [148]. It is well known that JA is essential for plants' capacity for adaptation and stress tolerance. It is also interesting to note that JA has a significant impact on increasing plants' resilience to environmental stressors [146]. For example, using JA boosted the grape seedlings' antioxidant defense system during HS. It has been proven that JA directly shields plants from HS when applied exogenously to wild-type plants before HS, reducing heat-induced unfavorable damage [10, 149]. However, the external application of 100 μM MeJA also mitigates HS by increasing sesquiterpene molecules in agarwood plants and restoring the endogenous JA pool [150, 151]. It also conclusively demonstrated the impact of methyl jasmonate (MeJA) treatment on electrolyte leakage in cell membranes under HS, providing clear evidence of the function of JA in heat tolerance in Arabidopsis [149]. On the contrary, using MeJA helped reduce the harmful effects of HS on cell viability, but treatment > 5 µm reversed this outcome by increasing membrane damage [149]. This suggests that MeJA's ability to mitigate the harmful effects of HS is concentration-specific [7]. Another study provided evidence about the impacts of jasmonic acid on ryegrass; it was discovered that methyl jasmonic acid (MeJA) improves plant tolerance to high temperatures by changing the defensive mechanism of antioxidants, reducing heat-induced decrease in chlorophyll, preserving proper water balance in the plant, and reducing crop electrolyte leakage [152, 153], also shown in Fig. 3. So, it creates a favorable effect on reducing heat-related stress situations.
4.4 Melatonin
N-acetyl-5-methoxytryptamine is a pleiotropic signaling molecule that regulates many environmental stressors, including HS [154]. Compared to glutathione and vitamin C, this molecule is a potent antioxidant and cellular protector [155]. Enhancing the detoxification of ROS through regulating antioxidant enzyme levels and activities is essential for improving plant stress tolerance [156]. Researchers have become interested in melatonin because it can protect against various environmental stressors.
Moreover, melatonin can stimulate germination, growth, and development activities as well as gene expression in response to abiotic and biotic stressors [157, 158]. Applying exogenous melatonin to transgenic plants raises their melatonin level, suppressing the breakdown of chlorophyll and reducing the CCE and SAG gene expression in response to various abiotic stressors [159, 160]. Melatonin is a powerful antioxidant and ROS scavenger that keeps excess ROS from building up and stops stress-induced senescence in plants [40, 161].
Melatonin has an influential role in preventing senescence-induced damage and gene expression in a variety of plant species, including model plants like Arabidopsis, fruits like grapes and kiwi, cereal crops like barley and rice, and also ryegrass and Chinese cabbage [159, 162, 163]. Researchers say that melatonin enhances the antioxidant defense mechanism and boosts flavonoid synthesis, thereby delaying the aging process in kiwifruit leaves [164, 165].
Several studies have examined the function of melatonin in heat-stress tolerance and found that exogenous melatonin treatment in tomato seedlings enhances the antioxidant defense system and improves its ability to withstand high temperatures [27, 166]. Moreover, melatonin was found to have a protective impact against HS in several different plant species, including radish [167], sweet potato [168], onion, leek [169], cucumber [170], kiwifruit [171] and maize [172]. According to Khan et al. [173], exogenous treatment with melatonin reduces HS effects in various tomato cultivars.
Foliar melatonin spraying enhances plant growth by enhancing antioxidant activities and photosynthetic pigment and guaranteeing the plants' resistance to heat, salt, drought, pathogens, heavy metals, cold stress, and acidic rain [174]. Plants under stress take advantage of melatonin's reduction of reactive oxygen species (H2O2, O2−) and enhancement of PSIII activity [175].
Although the effects of melatonin are well-established, its molecular mechanism of action is yet unknown. To optimize application techniques, research is needed to determine the best spraying rates and timings for various stressors. It is also unknown how potential effects might affect the good bacteria in the soil. A more thorough comprehension of the function of melatonin in particular stress reactions, such as heat or drought, may be beneficial. Lastly, a more comprehensive approach to plant health may result from investigating the interactions between melatonin and currently used stress management strategies.
4.5 Salicylic acid
A common chemical in plants, salicylic acid (SA), is essential to their resistance to various stressors. It performs the crucial signaling role of setting off a series of physiological reactions that allow plants to endure adverse environmental conditions and circumstances [176]. SA is a very promising chemical for lessening plant cytotoxicity and stresses caused by heat. By boosting their antioxidant capacity and regulating stress hormones, salicylic acid helps plants recover from the damaging effects of HS. In hot weather, this mitigation aids in preserving plant development and output [177, 178]. Stress-induced stress accumulation can occur locally in the root, where it is in direct contact with the stressor, but it can also travel to the tissue above ground. For instance, in barley, SA builds up in the roots but not in the shoots in response to dryness [179]. The synthesis of SA inside a plant's tissues rises dramatically in response to stressors such as pathogen assault, dehydration, or excessive salt. In addition, it is progressively transmitted from the roots to the shoots via the xylem in grapes exposed to HS and improved cold stress tolerance [180]. This spike in SA serves as a signal, setting off an intricate web of defenses. Activating the plant's innate immune system results in the synthesis of antimicrobial chemicals and the hypersensitive response, a process of localized cell death that limits the spread of pathogens. This is one of SA's main functions [180]. Furthermore, SA can induce the synthesis of proteins that respond to stress, such as antioxidants, which aid in scavenging dangerous free radicals produced under stressful circumstances (Fig. 3). Changes in endogenous SA levels brought on by stress vary depending on the species. Drought, for instance, raises the SA content in barley [179]. Moreover, SA can affect root architecture and stomatal conductance, which controls how leaves open and close, which may help with water management and nutrient intake in stressful situations. When chickpea plants experience minimal, moderate, or HS, administering SA (10 µM) to the plants increases, replenishes, and partially recovers root length in water level [181].
In summary, salicylic acid orchestrates a complex response to various environmental challenges in plants by acting as a critical signaling molecule. SA boosts plant stress tolerance and resilience by increasing antioxidant production, regulating growth and development, and activating defense mechanisms. These acts eventually contribute to plant survival and effective reproduction [182, 183]. This stress greatly influences plant behavior.
4.6 Strigolactones
Strigolactones are carotenoid derivatives with multiple functions and are newly recognized as crucial plant hormones [184]. According to Banerjee and Roychoudhury [185], they are essential in controlling several physiological processes, such as leaf senescence, flowering, seedling growth, efficient photosynthesis, and ion homeostasis.
Endogenous strigolactones act as endogenous growth regulators and enhance stress tolerance capacity in various horticultural crops [186]. They regulate multiple physiological and molecular processes in plant responses to abiotic stresses, serve as second messengers in shoot branching, and reduce auxin transport [187, 188]. In lupine, seed priming with strigolactones improves seed germination and seedling growth while reducing oxidative stress [189]. According to Zheng et al. [190], strigolactone application regulates the antioxidant defense system, increases nutrient uptake and reduces oxidative damage in cucumber under stress conditions.
Exogenous strigolactone application in plants has remarkably affected plant growth and stress response. Studies have shown that in Vitis vinifera, strigolactones not only enhance growth but also increase relative water content and improve the activity of antioxidant enzymes, gas exchange parameters and chlorophyll fluorescence under abiotic stress conditions [191]. Additionally, they mitigate oxidative injury and stomatal opening, highlighting their importance in stress mitigation.
Furthermore, strigolactones are vital in root architecture and arbuscular mycorrhizal symbiosis and enhance nutrient intake [192]. Though roots produce a small amount of strigolactones, they are synthesized in other plant parts [193]. Their impact extends to modifying root growth patterns in tomatoes and increasing the chlorophyll content and net photosynthetic rate under stress conditions in apple plants [190, 194].
While strigolactones enhance abiotic stress tolerance, their specific mechanisms for HS tolerance remain unclear [195, 196]. However, studies suggest their involvement in conferring heat-stress tolerance by modulating the activity of cell-cycle and auxin transport-related genes.
The processes underlying how they stand up to HS are still unknown despite their established ability to improve abiotic stress tolerance. Furthermore, there is a paucity of information regarding strigolactone responses to coupled stressors, such as salinity and heat or drought and heat. Since most studies are conducted in controlled settings, further field research is necessary to evaluate their usefulness for agriculture.
4.7 Gibberellins
Gibberellins regulate growth in several ways, including seed germination, stem elongation, fruit size increase, and inducing flowering [197, 198]. Also, gibberellin (GA), and indole-3-acetic acid (IAA), are hypothesized to play a role in controlling the growth of reproductive organs as well as processes related to pollination, fertilization, and spikelet fertility. However, these plant signaling hormones are important in mitigating HS conditions. For example, exogenous GA treatment has been shown to enhance the weight of individual fruits and increase the number of viable seeds and the activity of antioxidant enzymes in potato tubers [154, 199]. However, the external use of GA3 (50 μM) can effectively mitigate the suppression of germination and growth of seedlings influenced by HS. Conversely, enhanced HS tolerance in Arabidopsis is achieved by over-expressing GASA4, GA3-triggered gene from beechnut (Fagus sylvatica) [200]. According to a different investigation, regular and dwarf barley seedlings were treated under HS situations with GA3 and the GA3 inhibitor paclobutrazol, demonstrating the role of GA3 in controlling HS tolerance. The treated with GA3 normal and dwarf plants showed heat sensitivity; that is, they had higher ion leakage and less photosynthetic pigments than those treated with paclobutrazol under HS [7]. Also, it modifies the plastid structure and cell wall of Solanum lycopersicum L. to increase resistance to temperature stress [201]. Grain weight under heat is mediated by IAA, GA, ABA, and CTK [202]. For example, spikelet fertility in rice gibberellic acid is vital in HS conditions [68]. Similarly, increased grain sink activity under HS was caused by a GA3-mediated mechanism that conferred HS resistance in wheat via improving invertase activities [7].
4.8 Brassinosteroids
Brassinosteroids (BRs) are one of the unique polyhydroxylated steroid hormones synthesized in the plant endoplasmic reticulum [203, 204]. They are present in almost all plant organs and play vital roles in regulating plant growth and development, along with responses to several environmental stresses [205]. Specifically, brassinosteroids help plants adapt to stressed environmental conditions by mitigating abiotic and biotic stresses [206].
Heat-stress tolerance mediated by BRs has been documented in numerous plant species like barley [207], B. inermis [208], Vigna radiata [209], tomato [210] and Brassica napus [211]. In plants like rice, BRs have been shown to enhance chlorophyll content, seed production and photosynthetic activity under heat-stress conditions [212]. The mechanisms underlying brassinosteroids-mediated heat-stress tolerance involve optimizing various processes related to physiological and biochemical factors, such as enhancing photosynthetic efficiency, membrane stability and antioxidant system activation [209, 213]. Moreover, BR analogs have been reported to alleviate the adverse effects of HS on plant growth and photosynthesis activity [214].
BRs also interact with other signaling pathways, such as ethylene (ET), jasmonic acid (JA), salicylic acid (SA) and abscisic acid (ABA) to promote heat-stress tolerance [215]. Transcription factors such as BRI1-EMS SUPPRESSOR 1 (BES1) and BRASSINAZOLE RESISTANCE1 (BZR1) are very crucial in regulating brassinosteroids-targeted genes involved in heat-stress responses [216]. However, further research is required on the precise molecular mechanisms underlying brassinosteroids-mediated heat-stress tolerance and their crosstalk with other phytohormones [217].
Though the role of BRs in plant heat-stress tolerance is recognized, there are significant research gaps. The precise molecular mechanisms and interactions with other phytohormones, such as ethylene, jasmonic acid, salicylic acid, and abscisic acid, still need to be fully understood. Moreover, studies mainly focus on a selected number of plant species, and the information on the combined effects of BRs and other environmental stresses are limited. Most research is conducted under controlled conditions, necessitating long-term field studies to evaluate practical applications. Further research is needed on synthetic brassinosteroids and analogs.
Resolving these limitations may improve brassinosteroids' applicability in agriculture. Breeding crops with better heat-stress tolerance can be achieved by detailed molecular mechanism observation. Furthermore, integrated stress management strategies can also be applied. Advances in synthetic biology that may produce efficient BR analogs are developing new tools for crop resilience. Long-term field studies can establish sustainable practices crucial for adapting to climate change and ensuring food security.
4.9 Auxin
Considering natural substances, indole-3-acetic acid (IAA) has been studied the most as an active auxin. One of the main phytohormones, auxin, controls numerous aspects of the growth and development of a plant, as well as how it reacts to its surroundings [198]. However, auxins may facilitate modifying plants' response to salinity, drought, heavy metals, extremes in temperature, and flooding stressors [151]. The plant hormone auxin is crucial in HS-related changes in plant morphology, specifically in stem (hypocotyl) elongation and leaf movement [11]. On the other hand, adding NAA to rice crops raised the auxin concentration, which in turn caused the pollen to behave generally at high temperatures, resulting in appropriate fertilization and pollination. Other effects of this increased auxin concentration include proper pollen tube growth, pistil elongation, and flower stylar length. However, to reduce HT-induced pollen sterility, 10–5 M IAA was also applied as a foliar spray to barley [218, 219]. When wheat experienced HS, applying one µM of IAA was found to potentially increase grain number and yield by 6% to 8% [153, 220]. However, it depends on whether utilizing exogenous auxins during abiotic stress then improves photosynthetic activity, ionic homeostasis and water balance, root architecture, antioxidant defense, seed germination, and reproductive organ viability while reducing the harmful impacts of extremely high temperature.
4.10 Ethylene
The basic gaseous plant hormone ethylene has a wide-ranging and intricate effect on how plants react to stress. While ethylene is frequently linked to fruit ripening and senescence (aging), it also acts as a signaling molecule in response to several environmental stresses [221]. Environmental factors, such as HS, can substantially influence ethylene biosynthesis by changing the expression and activity of critical enzymes in the ethylene production pathway, such as ACC synthase and ACC oxidase (Fig. 3). These enzymes are activated in response to high temperatures, producing higher ethylene levels. This ethylene buildup acts as a signaling molecule, triggering adaptive responses to heat-induced harm, including growth regulation and defensive mechanism activation. The extensive regulatory networks and feedback loops that govern ethylene production highlight ethylene's crucial function in plant stress physiology [222, 223]. However, depending on the concentration, plant species, and particular stress experienced, its effects on stress tolerance might be contradictory [224]. Plants experience "stress ethylene," a sudden spike in production that occurs when they are under stress [225]. This increase in ethylene may have advantages as well as disadvantages. Ethylene can induce changes that improve stress tolerance. For example, it can increase the synthesis of protective substances like antioxidants, which aid in scavenging free radicals produced during stress and safeguarding cellular components [173].
Furthermore, ethylene may encourage root development in drought conditions and help with water and nutrient uptake [226]. On the other hand, there may be negative consequences if ethylene levels rise too high. Plant development can be inhibited, leaf senescence accelerated, and even programmed cell death triggered by ethylene [224]. This demonstrates the fine balance needed for ethylene to perform well in the stress response. To manage ethylene levels and improve plant resilience, it is essential to comprehend the precise routes and interactions between ethylene signaling and other stress response systems [222]. Ethylene works with the ROS response pathway to modify plant metabolism and the antioxidant system in response to heavy metal stressors and floods, hence increasing plant survival [227]. Plants use the reciprocal activation of auxin and ethylene signaling to improve root hair initiation and development to reduce the stress caused by magnesium shortage [228]. Under HS, preserving normal root development and gravity-sensing [229, 230]. Ethylene helps plants manage their water during dry times by encouraging root development and improving nutrient and water absorption. Because it raises a variety of stressors, accelerates leaf senescence, and lowers overall plant growth, ethylene is detrimental to plant growth and development. Furthermore, ethylene may cause large-scale programmed cell death, suggesting that it negatively impacts plants.
4.11 Gamma-aminobutyric acid (GABA)
Gamma-aminobutyric acid is a four-carbon non-protein amino acid in both autotrophic and heterotrophic organisms [231]. It plays various roles in plant physiology, particularly in response to stress conditions, including HS. Under higher temperatures, plants may exhibit multiple symptoms, including oxidative damage, chlorophyll degradation, ultrastructural changes and photoinhibition [232]. GABA initiates changes in the antioxidant defense system, the pathway of heat shock factor, and metabolic homeostasis, which enhances heat tolerance [233, 234]. Based on recent studies, applying GABA externally can strengthen plant seedlings' antioxidant defense mechanisms and shield them from HS [235, 236]. A recent study on the creeping bentgrass leaf found that GABA enhances heat tolerance by regulating metabolic homeostasis, tricarboxylic acid cycle, and osmotic potential [235]. GABA can also considerably increase the amount of HSP101, HSP70 and HSP901 in creeping bentgrass leaves and the expression of heat-induced HSPs and HSFs [237]. In addition, GABA increases the antioxidant capacity, vitality and osmotic adjustment of roots, improving their ability to respond to HS. Furthermore, GABA controls heat-stressed metabolites by improving cellular architecture, antioxidant capacity, root osmotic balance and energy metabolism [238]. Moreover, GABA functions as a link to control plants' carbon and nitrogen ratio and contributes to their tolerance to various stressors. The build-up of GABA strengthens the plant's resistance to oxidative stress, reducing excess ROS in the cells [239].
5 Effect nutrients on mitigation of heat stress
Nutrients are essential for mitigating plant HS because they support critical physiological functions that help plants cope with high temperatures (Fig. 4). These include controlling the opening of stomata to prevent water loss, maintaining the cell membrane integrity, triggering the activity of enzymes connected to stress response processes, and strengthening antioxidant defense systems. Heat stress damages root hair mass through which plants uptake nutrients. External nutrients decrease root damage and help in the passive transport of nutrients. Ca, N, Fe, P, B, S & Se are transported through CDPKs, NRT-1 & 2, IRT- 1, PHT-1, BOR-1, SULTR- 1 & 2 transporters (Fig. 4). As a result, plants experience various physiological and biochemical changes that mitigate the effect of heat stress. Numerous macro and micronutrients influence numerous physiological and biochemical processes to reduce HS. For an instance,
Nitrogen (N): Plants' ability to withstand HS can be effectively increased with the right amount of nitrogen [240]. One essential element of chlorophyll is nitrogen and limited nitrogen can accelerate photosynthesis by extending the area of leaves, thus improving the absorbance of light energy [241, 242]. Increased Nitrogen application enhances the leaves' nitrogen metabolism and minimizes high-temperature damage [240, 243]. Conversely, enhanced photosynthesis may stimulate nitrogen metabolism, producing sufficient nutrients and raising fruit quality and yield [243, 244]—additionally, nitrogen aids in germination. When seedlings germinate in standard and high-stress conditions, nitrate priming enhances seed germination and crop growth. It also allows plants to revert to HS-associated reactions while retaining their potential yield [245].
Potassium (K): Chlorophyll content, enzymatic activity, stomatal conductance, transpiration rate, antioxidant levels, and photosynthesis are all reduced in heat-stressed plants [18]. A plant system's fundamental physiological and metabolic mechanisms depend on potassium [246, 247]. Foliar spray of potassium silicate significantly mitigated HS-induced effects [248]. It can function as a biostimulant to increase standing crop biomass and boost performance associated with photosynthesis [249] and also improves stomatal conductance and the leaf cortical wax layer, which are beneficial in heat-stressed conditions [250]. Overall, the adverse effects of HS on plant development and productivity can be considerably reduced by ensuring adequate potassium levels.
Calcium (Ca): Calcium plays a crucial role in plant development and helps mitigate HS in several ways. Firstly, it helps in stabilizing cell membranes and enhances the interaction between phytohormones and enzymes during abiotic stress [251]. Additionally, calcium regulates the process of stomata opening and closing [252], which helps control water loss during HS. Moreover, stress-responsive genes in plants can be activated by Ca2+, which functions as a secondary transmitter in signaling pathways [253, 254]. Additionally, sufficient calcium levels are necessary for both normal physiological processes and the preservation of plants' structural integrity [254, 255].
Magnesium (Mg): A sufficient magnesium supply promotes the activities of antioxidant enzymes, reducing cellular damage caused by ROS. This boosts plant growth, development, and resilience to high temperatures [39]. It promotes root development and surface area and aids in activating chloroplast enzymes, a preliminary enzyme of photosynthesis, resulting in higher water and nutrient uptake capacity [255,256,257], which is beneficial for energy production and overall plant growth. Additionally, magnesium aids in photosynthesis by enlarging the leaf surface to absorb solar radiation [39]. Fertilizing crops with sufficient amounts of magnesium reduces heat-related loss in crop production [258].
Boron (B): Structural instability can occur due to cell wall damage in HS conditions. Exogenous application of boron reinforces cell walls, helping plants maintain their structural integrity and spikelet fertility, which minimizes the damage caused by high temperatures [259]. Also, foliar application of B positively affects wheat's biochemical and physiological characteristics [260] and rice [259] under HS. In addition, the approximate amount of foliar spray of boron during HS enhances PSII photochemical efficiency and decreases plant oxidative damage under HS [261].
Zinc (Zn): Zn is an essential micronutrient that helps mitigate HS in plants by regulating various physiological and biochemical processes. Zinc improves antioxidant enzyme activity, which scavenges ROS generated under HS [262]. Furthermore, the foliar application of zinc aids in maintaining the structural integrity of cell membranes and protects photosynthesis, thus preventing heat-induced damage [263]. Overall, adequate zinc levels are essential for plants to cope with HS effectively.
Selenium (Se): Selenium helps plants mitigate HS by enhancing their antioxidant defense system. It scavenges ROS, reduces oxidative damage and boosts the activity of antioxidant enzymes [264, 265], thereby increasing the plant's resistance to extreme temperatures. There are various types of selenium, such as seleniumate, nano selenium (nano-Se), selenioproteins and selenium selenite [266]. Se and nSe can mitigate oxidative stress, thus reducing abiotic stresses, including HS [267]. Se enhances photorespiration, protects chlorophyll, and maintains photosynthesis [268]. Under HS at 40 °C, Se nanoparticles (SeNPs) enhance photosynthetic activities and raise the chlorophyll of various Chrysanthemum morifolium Ramat cultivars [269].
6 Role of exogenous phytohormones and plant nutrients on sustainable agriculture
6.1 Plant nutrients
Exogenous plant nutrients play a pivotal role in sustainable agriculture by enhancing soil health, plant growth, and disease resistance. Sustainable agriculture aims to maintain the agricultural ecosystems' productivity and biodiversity, fulfilling ecological, economic, and social functions without harming other ecosystems. Integrative plant nutrition is essential in sustainable agriculture, offering a cost-effective and environmentally friendly disease control method [270]. These nutrients, including macronutrients like N, P, and K, and micronutrients such as Mn, Zn, boron (B), Cl, and Si, are crucial for maintaining plant vitality and productivity. Nitrogen is essential for amino acid and chlorophyll production, influencing plant metabolism and growth. Phosphorus, though variable in its effects, is vital for energy transfer and genetic material synthesis. Potassium improves plant resistance to diseases up to an optimal level. Micronutrients like Mn, Zn, and B contribute to lignin and phenol biosynthesis, membrane stability, and metabolic functions, enhancing disease resistance [270, 271]. Chlorine aids in osmotic regulation, while silicon forms a physical barrier against pathogens and induces antifungal compound accumulation [272]. However, the mismanagement of fertilizer nutrients has significantly impacted soil productivity, resulting in several nutrient deficiencies, groundwater pollution, reduced crop yields, and a threat to world food security [273]. It is essential to apply plant nutrients carefully and thoughtfully, which can significantly enhance crop yields and promote the long-term sustainability of agricultural systems. The application of biofertilizers, which consist of living microorganisms, has gained prominence in organic farming. These biofertilizers, including plant growth-promoting rhizobacteria (PGPR) and fungi (PGPF), facilitate nutrient solubilization and mineralization, thus enriching soil fertility and plant health. PGPR and PGPF produce phytohormones like auxins, gibberellins, cytokinins, and abscisic acid, promoting root and shoot growth while offering heat stress tolerance through mechanisms like induced Systemic resistance (ISR) and Systemic acquired resistance (SAR) [274, 275]. The synergy between plants and soil microbes is crucial for sustainable agriculture, as it leads to improved soil structure, nutrient availability, and crop resilience against environmental stresses [276, 277].
6.2 Phytohormones
The most significant endogenous compounds in the regulation of physiological and molecular responses that guarantee plants' survival in harsh environments are thought to be phytohormones. The diverse functions of phytohormones, essential plant growth and development regulators, in strengthening crop resilience against environmental stressors are increasingly recognized [278]. From the start of seed germination to its maturity, auxins, gibberellins, cytokinins, ABA, salicylic acids, JAs and BRs coordinate every step of ontogenesis [279]. Initially, exogenous phytohormone administration greatly reduced heat-induced damage and increased plant heat tolerance, suggesting that phytohormones play a role in the plant's ability to respond to heat stress [10]. The vitality and resilience of plants in their constantly changing settings depend on plant hormones, which are essential for coordinating and adjusting these factors to guarantee optimal growth and adaptation to changing situations. In sustainable agriculture, phytohormones are essential because they provide ways to improve crop output and resilience to stress without resorting to traditional chemical treatments [278]. However, to reduce yield losses, modulating endogenous phytohormones or applying exogenous phytohormones may be potential strategies for plant adaptation and recovery from HS [153]. On the other hand, heat-induced thermomorphogenesis in plants is mediated by auxin synthesis, sensing, and signalling pathways; hence, auxin provides an opportunity to create climate-smart plants to guarantee crop and food productivity in the context of global climate change [10]. Applying exogenous phytohormones has also been suggested as a practical strategy to deal with various abiotic stressors, including salinity, drought, and heat [1]. First of all, exogenous treatment of ascorbic acid (70 ppm), moringa leaf extract, H2O2 (30 ppm), and SA (50 ppm) increased cotton's net photosynthetic rate and yield-contributing features while also shielding the plants from heat-induced damage by modifying the antioxidant enzyme mechanism [7]. When plant hormones are applied externally, a variety of genes are triggered, which enables the development of tolerance in the plants. That is what helps achieve the goal of sustainable agriculture. For example, methyl jasmonic acid (MeJA) improves plant tolerance to high temperatures by modifying the antioxidant defense system, reducing heat-induced chlorophyll loss, preserving the plant's water balance, and reducing crop electrolyte leakage. This mechanism also helps to increase the yields of different crop plants. Second, rice reduced the adverse effects of high-temperature stress by applying PGRs. Compared to NAC, the exogenous application of PGRs significantly enhanced the growth and yield of both rice varieties [153]. Another example is exogenous IAA, a useful tool for controlling crop growth and development and increasing yields. When wheat plants were treated with IAA solution in the field, their growth was improved, their morphological characteristics (plant height, flag leaf area, spike length, number of spikes, and grain in spike) improved, and their yield increased [279]. Applying exogenous GA treatment also offers additional advantages, such as encouraging early and abundant sprouting in potato tubers, increasing the weight of individual fruits, and enhancing the number of viable seeds and antioxidant enzyme activity [280]. The circuit's regulatory elements could be useful targets for genetic engineering and molecular breeding to create heat-resistant crops that would ensure food production in the future [10]. So, exogenous phytohormones play an important role in achieving sustainable agriculture. It should be mentioned that synthetic plant growth regulators are mostly utilized in agriculture; their synthesis comes at a significant expense, and their use adds to the existing chemical pollution. Verifying their biological safety for humans and animals is costly and, more importantly, time-consuming. Thus, it is becoming increasingly crucial to develop techniques for extracting physiologically active compounds for use in agriculture from naturally occurring biological raw materials that contain phytohormones.
7 Present study’s limitations
We could not access informations from numerous locked papers for our present study. Many studies have been conducted on the effects of phytohormones under heat stress, whereas the impacts of nutrients have received very little attention. Therefore, gathering information on this subject was difficult. We also excluded studies outside our criteria, as we selected some unique criteria for this review.
8 Research gap and future perspective
One significant area of unfilled research need is the lack of knowledge of the genetic and molecular processes underlying heat tolerance. This offers a chance for future studies to create focused strategies to increase crop resistance to rising global temperatures. It is poorly understood how phytohormones control the defensive response at the molecular level. The chemical transduction route that triggers the production of hormones at elevated temperatures is still elusive [10]. The unpredictability of the effects of phytohormone on ecosystems and non-target creatures makes environmental impact assessment imperative. A thorough application of phytohormones on crops can affect humans' gut microbiota, metabolism, and nutrition [281].
More research is required to fully understand the complex relationships and unique mechanisms by which these phytohormones regulate plant development and environmental adaptability. Soon, we will work on this topic to discover the processes that enhance seed germination, subsequent plant growth, and development following exogenous phytohormone priming, which are little investigated and unclear. Also, long-term research conducted in various agricultural settings is needed to guarantee a thorough comprehension of the applications of phytohormone alterations.
Using incompatible pesticides may compromise phytohormones' efficacy, necessitating a strong regulatory framework [282]. It is crucial to inform consumers and producers about these strategies. Commercializing phytohormones as possible agrochemicals will open up new opportunities for financial gain, health advantages, and environmental sustainability in the future, making them significant contributors to the advancement of sustainable agribusiness [283, 284].
Optimizing gene alterations for regulated expression should be the top priority for future phytohormone engineering research. To precisely control the timing and amount of plant hormone production, minute modifications to regulatory components are required. Investigating promoters that are conditional, stress-triggered, or senescence-induced is essential to preventing phytohormones' unfavorable impacts on plant growth. These promoters enable more sophisticated regulation of gene expression by triggering the synthesis of phytohormones only in particular developmental phases or environmental contexts.
Extensive research is required on certain nutritional combinations with particular phytohormones. Some nutrients accelerate the process by which those particular phytohormones function. This requires finance, skilled personnel, and well-equipped labs. Furthermore, it is crucial to comprehend the complex interactions among phytohormone signaling pathways while making genetic alterations. Examining the interactions and effects of distinct phytohormones on one another's expression might yield important information about optimizing plants that exhibit desirable characteristics (Ali et al., 2024).
The consequences of phytohormone engineering must be studied in real-world situations with variable field circumstances, not just in controlled laboratory settings. Research needs to be conducted across varied agricultural contexts to ensure a thorough grasp of the practical effects of phytohormone changes. In addition to resolving issues related to climate, soil, and the threat of pests' unpredictability, this strategy is crucial for guaranteeing sustainable and predictable results in agriculture.
Data availability
No datasets were generated or analysed during the current study.
References
Resentini F, Orozco-Arroyo G, Cucinotta M, Mendes MA. The impact of heat stress in plant reproduction. Front Plant Sci. 2023;14:1271644.
Bita CE, Gerats T. Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci. 2013;4:273.
Ihsan MZ, Daur I, Alghabari F, Alzamanan S, Rizwan S, Ahmad M, Waqas M, Shafqat W. Heat stress and plant development: role of sulphur metabolites and management strategies. Acta Agric Scand Section B Soil Plant Sci. 2019;69(4):332–42. https://doi.org/10.1080/09064710.2019.1569715.
Alghabari F, Ihsan MZ, Khaliq A, Hussain S, Daur I, Fahad S, Nasim W. Gibberellin-sensitive Rht alleles confer tolerance to heat and drought stresses in wheat at booting stage. J Cereal Sci. 2016;70:72–8.
Lobell DB, Bänziger M, Magorokosho C, Vivek B. Nonlinear heat effects on African maize as evidenced by historical yield trials. Nat Clim Chang. 2011;1(1):42–5.
Fahad S, Hussain S, Saud S, Hassan S, Chauhan BS, Khan F, Ihsan MZ, Ullah U, Wu C, Bajwa AA, Alharby H, Amanullah, Nasim W, Shahzad B, Tanveer M, Huang J. Responses of rapid viscoanalyzer profile and other rice grain qualities to exogenously applied plant growth regulators under high day and high night temperatures. PLoS ONE. 2016;11(7): e0159590.
Jha UC, Nayyar H, Siddique KHM. Role of phytohormones in regulating heat stress acclimation in agricultural crops. J Plant Growth Regul. 2022;41(3):1041–64. https://doi.org/10.1007/s00344-021-10362-x.
Waswani H, Ranjan R. Phytohormones as stress mitigators in plants. In Mineral biofortification in crop plants for ensuring food security. Singapore: Springer Nature Singapore; 2023;19–338
Jahan MS, Wang Y, Shu S, Zhong M, Chen Z, Wu J, Sun J, Guo S. Exogenous salicylic acid increases the heat tolerance in Tomato (Solanum lycopersicum L) by enhancing photosynthesis efficiency and improving antioxidant defense system through scavenging of reactive oxygen species. Sci Hortic. 2019;247:421–9.
Li N, Euring D, Cha JY, Lin Z, Lu M, Huang LJ, Kim WY. Plant hormone-mediated regulation of heat tolerance in response to global climate change. Front Plant Sci. 2021;11: 627969.
Küpers JJ, Oskam L, Pierik R. Photoreceptors regulate plant developmental plasticity through auxin. Plants. 2020;9(8):940.
Grover A, Mittal D, Negi M, Lavania D. Generating high temperature tolerant transgenic plants: achievements and challenges. Plant Sci. 2013;205:38–47.
Sadiq Y, Zaid A, Khan MMA. Adaptive physiological responses of plants under abiotic stresses: role of phytohormones. Springer eBooks. 2020. https://doi.org/10.1007/978-981-15-2156-0_28
Piacenza L, Zeida A, Trujillo M, Radi R. The superoxide radical switch in the biology of nitric oxide and peroxynitrite. Physiol Rev. 2022;102(4):1881–906.
Kumar D, Ohri P. Say “NO” to plant stresses: unravelling the role of nitric oxide under abiotic and biotic stress. Nitric Oxide. 2023;130:36–57.
Sardans J, Peñuelas J. Potassium control of plant functions: Ecological and agricultural implications. Plants. 2021;10(2):419.
Hu S, Ding Y, Zhu C. Sensitivity and responses of chloroplasts to heat stress in plants. Front Plant Sci. 2020;11: 520328.
Hussain HA, Hussain S, Khaliq A, Ashraf U, Anjum SA, Men S, Wang L. Chilling and drought stresses in crop plants: implications, cross talk, and potential management opportunities. Front Plant Sci. 2018;9:393.
Sattar A, Sher A, Ijaz M, Ul-Allah S, Rizwan MS, Hussain M, Jabran K, Cheema MA. Terminal drought and heat stress alter physiological and biochemical attributes in flag leaf of bread wheat. PLoS ONE. 2020;15(5): e0232974.
Mukhtar T, Rehman SU, Smith D, Sultan T, Seleiman MF, Alsadon AA, Amna, Ali S, Chaudhary HJ, Solieman THI, et al. Mitigation of heat stress in Solanum lycopersicum L. by ACC-deaminase and exopolysaccharide producing Bacillus cereus: effects on biochemical profiling. Sustainability. 2020;12(6):2159.
Shen HF, Zhao B, Xu JJ, Liang W, Huang WM, Li HH. Effects of heat stress on changes in physiology and anatomy in two cultivars of Rhododendron. S Afr J Bot. 2017;112:338–45.
Yang D, Peng S, Wang F. Response of photosynthesis to high growth temperature was not related to leaf anatomy plasticity in rice (Oryza sativa L.). Front Plant Sci. 2020;11:26.
Yuan L, Tang L, Zhu S, Hou J, Chen G, Liu F, Wang C. Influence of heat stress on leaf morphology and nitrogen–carbohydrate metabolisms in two wucai (Brassica campestris L.) genotypes. Acta Societatis Botanicorum Poloniae, 2017;86(2).
Rossi S, Burgess P, Jespersen D, Huang B. Heat-induced leaf senescence associated with chlorophyll metabolism in bentgrass lines differing in heat tolerance. Crop Sci. 2017;57(S1):169.
Mustafa T, Sattar A, Sher A, Ul-Allah S, Ijaz M, Irfan M, Butt M, Cheema M. Exogenous application of silicon improves the performance of wheat under terminal heat stress by triggering physio-biochemical mechanisms. Sci Rep. 2021;11(1):23170.
Wassie M, Zhang W, Zhang Q, Ji K, Cao L, Chen L. Exogenous salicylic acid ameliorates heat stress-induced damages and improves growth and photosynthetic efficiency in alfalfa (Medicago sativa L.). Ecotoxicol Environ Saf. 2020;191: 110206.
Jahan MS, Shu S, Wang Y, Chen Z, He M, Tao M, Sun J, Guo S. Melatonin alleviates heat-induced damage of tomato seedlings by balancing redox homeostasis and modulating polyamine and nitric oxide biosynthesis. BMC Plant Biol. 2019;19:1–16.
Afzal I, Akram MW, Rehman HU, Rashid S, Basra SMA. Moringa leaf and sorghum water extracts and salicylic acid to alleviate impacts of heat stress in wheat. S Afr J Bot. 2020;129:169–74.
Rashid N, Khan S, Wahid A, Basra SMA, Alwahibi MS, Jacobsen SE. Impact of natural and synthetic growth enhancers on the productivity and yield of quinoa (Chenopodium quinoa willd.) cultivated under normal and late sown circumstances. J Agron Crop Sci. 2022;208(4):552–66.
Lakshmi G, Beena R, Soni KB, Viji MM, Jha UC. Exogenously applied plant growth regulator protects rice from heat-induced damage by modulating plant defense mechanism. J Crop Sci Biotechnol. 2023;26(1):63–75. https://doi.org/10.1007/s12892-022-00162-4.
Anderson CM, Mattoon EM, Zhang N, Becker E, McHargue W, Yang J, Patel D, Dautermann O, McAdam SAM, Tarin T, Pathak S, Avenson TJ, Berry J, Braud M, Niyogi KK, Wilson M, Nusinow DA, Vargas R, Czymmek KJ, Eveland AL, Zhang R. High light and temperature reduce photosynthetic efficiency through different mechanisms in the C4 model Setaria viridis. Commun Biol. 2021;4(1):1092.
Zhang J, Li H, Huang X, Xing J, Yao J, Yin T, Jiang J, Wang P, Xu B. STAYGREEN-mediated chlorophyll a catabolism is critical for photosystem stability during heat-induced leaf senescence in perennial ryegrass. Plant Cell Environ. 2022;45(5):1412–27.
Cai YQ, Tarin MWK, Fan LL, Xie DJ, Rong JD, He TY, Chen LG, Zheng YS. Responses of photosynthesis, chloroplast ultrastructure, and antioxidant system of Morinda officinalis how to exogenous 2, 4-epibrassinolide treatments under high temperature stress. Appl Ecol Environ Res. 2020;18(3).
Wang QL, Chen JH, He NY, Guo FQ. Metabolic reprogramming in chloroplasts under heat stress in plants. Int J Mol Sci. 2018;19(3):849.
Liu DF, Zhang D, Liu GQ, Hussain S, Teng YW. Influence of heat stress on leaf ultrastructure, photosynthetic performance, and ascorbate peroxidase gene expression of two pear cultivars (Pyrus pyrifolia). J Zhejiang Univ Sci B. 2013;14:1070–83.
Munir MU, Shabbir GH. Salicylic acid mediated heat stress tolerance in selected bread wheat genotypes of Pakistan. Pak J Bot. 2018;50(6):2141–6.
Rashid N, Basra SM, Shahbaz M, Iqbal S, Hafeez MB. Foliar applied moringa leaf extract induces terminal heat tolerance in quinoa. Int J Agric Biol. 2018;20(1):157–64.
Batool AS, Wahid AB, Abbas G, Shah SH, Naeem MU, Akhtar NP, Hassnain ZU. Application of Moringa oleifera plant extracts for enhancing the concentration of photosynthetic pigments leading to stable photosynthesis under heat stress in maize (Zea mays L.). Pak J Bot. 2019;51(6):2031–6.
Siddiqui MH, Alamri SA, Al-Khaishany MY, Al-Qutami MA, Ali HM, Al-Whaibi MH, Al-Whaibi MS, Alharby HF. Mitigation of adverse effects of heat stress on Vicia faba by exogenous application of magnesium. Saudi J Biol Sci. 2018;25(7):1393–401.
Ahammed GJ, Xu W, Liu A, Chen S. COMT1 silencing aggravates heat stress-induced reduction in photosynthesis by decreasing chlorophyll content, photosystem II activity, and electron transport efficiency in tomato. Front Plant Sci. 2018;9: 386258.
Jiang Y, Feng X, Wang H, Chen Y, Sun Y. Heat-induced down-regulation of photosystem II protects photosystem I in honeysuckle (Lonicera japonica). J Plant Res. 2021;134:1311–21.
Medina E, Kim SH, Yun M, Choi WG. Recapitulation of the function and role of ROS generated in response to heat stress in plants. Plants. 2021;10(2):371.
Distéfano AM, Martin MV, Córdoba JP, Bellido AM, D’Ippólito S, Colman SL, Soto D, Roldán JA, Bartoli CG, Zabaleta EJ, Fiol DF, Stockwell BR, Dixon SJ, Pagnussat GC. Heat stress induces ferroptosis-like cell death in plants. J Cell Biol. 2017;216(2):463–76.
Katano K, Oi T, Suzuki N. Failure of pollen attachment to the stigma triggers elongation of stigmatic papillae in Arabidopsis thaliana. Front Plant Sci. 2020;11:989.
Muhlemann JK, Younts TL, Muday GK. Flavonols control pollen tube growth and integrity by regulating ROS homeostasis during high-temperature stress. Proc Natl Acad Sci. 2018;115(47):E11188–97.
Paupière MJ, Müller F, Li H, Rieu I, Tikunov YM, Visser RG, Bovy AG. Untargeted metabolomic analysis of tomato pollen development and heat stress response. Plant Reprod. 2017;30:81–94.
Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biol Med. 1996;20(7):933–56.
Chen D, Shao Q, Yin L, Younis A, Zheng B. Polyamine function in plants: metabolism, regulation on development, and roles in abiotic stress responses. Front Plant Sci. 2019;9:1945.
Mittler R. ROS are good. Trends Plant Sci. 2017;22(1):11–9.
Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. 2012. https://doi.org/10.1155/2012/217037.
Hu L, Liang W, Yin C, Cui X, Zong J, Wang X, Hu J, Zhang D. Rice MADS3 regulates ROS homeostasis during late anther development. Plant Cell. 2011;23(2):515–33.
Suzuki N, Katano K. Coordination between ROS regulatory systems and other pathways under heat stress and pathogen attack. Front Plant Sci. 2018;9: 351917.
Suzuki N, Mittler R. Reactive oxygen species and temperature stresses: a delicate balance between signaling and destruction. Physiol Plant. 2006;126(1):45–51.
Zhao Q, Zhou L, Liu J, Cao Z, Du X, Huang F, Pan G, Cheng F. Involvement of CAT in the detoxification of HT-induced ROS burst in rice anther and its relation to pollen fertility. Plant Cell Rep. 2018;37:741–57.
Ali MGM, Ahmed M, Ibrahim MM, El Baroudy AA, Ali EF, Shokr MS, Aldosari AA, Majrashi A, Kheir AMS. Optimizing sowing window, cultivar choice, and plant density to boost maize yield under RCP8.5 climate scenario of CMIP5. Int J Biometeorol. 2022;66(5):971–85. https://doi.org/10.1007/s00484-022-02253-x.
Shaffique S, Khan MA, Wani SH, Pande A, Imran M, Kang S-M, Rahim W, Khan SA, Bhatta D, Kwon E-H, Lee I-J. A review on the role of endophytes and plant growth promoting rhizobacteria in mitigating heat stress in plants. Microorganisms. 2022;10(7):1286. https://doi.org/10.3390/microorganisms10071286.
Firmansyah, Argosubekti N. A review of heat stress signaling in plants. IOP Confer Series: Earth Environ Sci. 2020;484(1): 012041. https://doi.org/10.1088/1755-1315/484/1/012041.
Alsamir M, Mahmood T, Trethowan R, Ahmad N. An overview of heat stress in tomato (Solanum lycopersicum L.). Saudi J Biol Sci. 2021;28(3):1654–63. https://doi.org/10.1016/j.sjbs.2020.11.088.
Kumari P, Rastogi A, Yadav S. Effects of heat stress and molecular mitigation approaches in orphan legume. Chickpea Mol Biol Rep. 2020;47(6):4659–70. https://doi.org/10.1007/s11033-020-05358-x.
Li M, Jannasch AH, Jiang Y. Growth and hormone alterations in response to heat stress in perennial ryegrass accessions differing in heat tolerance. J Plant Growth Regul. 2020;39(3):1022–9. https://doi.org/10.1007/s00344-019-10043-w.
Fan Y, Ma C, Huang Z, Abid M, Jiang S, Dai T, Zhang W, Ma S, Jiang D, Han X. Heat priming during early reproductive stages enhances thermo-tolerance to post-anthesis heat stress via improving photosynthesis and plant productivity in winter wheat (Triticum aestivum L.). Front Plant Sci. 2018;9:805. https://doi.org/10.3389/fpls.2018.00805.
Hassan MU, Chattha MU, Khan I, Chattha MB, Barbanti L, Aamer M, Iqbal MM, Nawaz M, Mahmood A, Ali A, Aslam MT. Heat stress in cultivated plants: nature, impact, mechanisms, and mitigation strategies—a review. Plant Biosyst. 2021;155(2):211–34. https://doi.org/10.1080/11263504.2020.1727987.
Lysenko EA, Kozuleva MA, Klaus AA, Pshybytko NL, Kusnetsov VV. Lower air humidity reduced both the plant growth and activities of photosystems I and II under prolonged heat stress. Plant Physiol Biochem. 2023;194:246–62. https://doi.org/10.1016/j.plaphy.2022.11.016.
Iqbal M, Raja NI, Mashwani Z-U-R, Hussain M, Ejaz M, Yasmeen F. Effect of silver nanoparticles on growth of wheat under heat stress. Iran J Sci Technol Trans A: Sci. 2019;43(2):387–95. https://doi.org/10.1007/s40995-017-0417-4.
Khalil U, Ali S, Rizwan M, Rahman KU, Ata-Ul-Karim ST, Najeeb U, Ahmad MN, Adrees M, Sarwar M, Hussain SM. Role of mineral nutrients in plant growth under extreme temperatures. In: Hasanuzzaman M, Fujita M, Oku H, Nahar K, Hawrylak-Nowak B, editors. Plant nutrients and abiotic stress tolerance. Singapore: Springer; 2018. p. 499–524. https://doi.org/10.1007/978-981-10-9044-8_21.
Poór P, Nawaz K, Gupta R, Ashfaque F, Khan MIR. Ethylene involvement in the regulation of heat stress tolerance in plants. Plant Cell Rep. 2022;41(3):675–98. https://doi.org/10.1007/s00299-021-02675-8.
Chaturvedi P, Wiese AJ, Ghatak A, Zaveska Drabkova L, Weckwerth W, Honys D. Heat stress response mechanisms in pollen development. New Phytol. 2021;231(2):571–85.
Wu C, Tang S, Li G, Wang S, Fahad S, Ding Y. Roles of phytohormone changes in the grain yield of rice plants exposed to heat: a review. PeerJ. 2019;7: e7792.
Carrizo García C, Nepi M, Pacini E. It is a matter of timing: asynchrony during pollen development and its consequences on pollen performance in angiosperms—a review. Protoplasma. 2017;254:57–73.
Hedhly A. Sensitivity of flowering plant gametophytes to temperature fluctuations. Environ Exp Bot. 2011;74:9–16.
Parrotta L, Faleri C, Cresti M, Cai G. Heat stress affects the cytoskeleton and the delivery of sucrose synthase in tobacco pollen tubes. Planta. 2016;243:43–63.
Zinn KE, Tunc-Ozdemir M, Harper JF. Temperature stress and plant sexual reproduction: uncovering the weakest links. J Exp Bot. 2010;61(7):1959–68.
De Storme N, Geelen D. High temperatures alter cross-over distribution and induce male meiotic restitution in Arabidopsis thaliana. Commun Biol. 2020;3(1):187.
Pecrix Y, Rallo G, Folzer H, Cigna M, Gudin S, Le Bris M. Polyploidization mechanisms: temperature environment can induce diploid gamete formation in Rosa sp. J Exp Bot. 2011;62(10):3587–97.
De Storme N, Geelen D. Cytokinesis in plant male meiosis. Plant Signal Behav. 2013;8(3): e23394.
Hafidh S, Fíla J, Honys D. Male gametophyte development and function in angiosperms: a general concept. Plant Reprod. 2016;29:31–51.
Begcy K, Nosenko T, Zhou LZ, Fragner L, Weckwerth W, Dresselhaus T. Male sterility in maize after transient heat stress during the tetrad stage of pollen development. Plant Physiol. 2019;181(2):683–700.
Jiang J, Liu X, Liu C, Liu G, Li S, Wang L. Integrating omics and alternative splicing reveals insights into grape response to high temperature. Plant Physiol. 2017;173(2):1502–18.
Jiang Y, Lahlali R, Karunakaran C, Warkentin TD, Davis AR, Bueckert RA. Pollen, ovules, and pollination in pea: success, failure, and resilience in heat. Plant, Cell Environ. 2019;42(1):354–72.
Wang J, Li D, Shang F, Kang X. High temperature-induced production of unreduced pollen and its cytological effects in Populus. Sci Rep. 2017;7(1):5281.
Płażek A, Słomka A, Kopeć P, Dziurka M, Hornyák M, Sychta K, Pastuszak J, Dubert F. Effects of high temperature on embryological development and hormone profile in flowers and leaves of common buckwheat (Fagopyrum esculentum Moench). Int J Mol Sci. 2019;20(7):1705.
Jegadeesan S, Chaturvedi P, Ghatak A, Pressman E, Meir S, Faigenboim A, Rutley N, Beery A, Harel A, Weckwerth W, Firon N. Proteomics of heat-stress and ethylene-mediated thermotolerance mechanisms in tomato pollen grains. Front Plant Sci. 2018;9:1558.
Cui X. Beyond yield response: weather shocks and crop abandonment. J Assoc Environ Resour Econ. 2020;7(5):901–32.
Gammans M, Mérel P, Ortiz-Bobea A. Negative impacts of climate change on cereal yields: statistical evidence from France. Environ Res Lett. 2017;12(5): 054007.
Talukdar P, Alagirusamy R, Das A. Heat transfer analysis and second degree burn prediction in human skin exposed to flame and radiant heat using dual phase lag phenomenon. Int J Heat Mass Transf. 2014;78:1068–79.
Chukwudi UP, Mavengahama S, Kutu FR. Relationships between grain weight and other yield component traits of maize varieties exposed to heat-stress and combined heat- and water-stress conditions. Stresses. 2022;2(4):467–76.
Zhao C, Liu B, Piao S, Wang X, Lobell DB, Huang Y, Huang M, Yao Y, Bassu S, Ciais P, Durand J-L, Elliott J, Ewert F, Janssens IA, Li T, Lin E, Liu Q, Martre P, Müller C, Peng S, Peñuelas J, Ruane AC, Wallach D, Wang T, Wu D, Liu Z, Zhu Y, Zhu Z, Asseng S. Temperature increase reduces global yields of major crops in four independent estimates. Proc the Natl Acad Sci. 2017;114(35):9326–31.
Al Masruri MHK, Ullah A, Farooq M. Application of nano chitosan-glycinebetaine for improving bread wheat performance under combined drought and heat stresses. J Soil Sci Plant Nutr. 2023;23(3):3482–99.
Kamara MM, Ibrahim KM, Mansour E, Kheir AMS, Germoush MO, Abd El-Moneim D, Motawei MI, Alhusays AY, Farid MA, Rehan M. Combining ability and gene action controlling grain yield and its related traits in bread wheat under heat stress and normal conditions. Agronomy. 2021;11(8):1450.
Roy C, Chattopadhyay T, Ranjan RD, Ul Hasan W, Kumar A, De N. Association of leaf chlorophyll content with the stay-green trait and grain yield in wheat grown under heat stress conditions. Czech J Genet Plant Breed. 2021;57(4):140–8.
Shenoda JE, Sanad MNME, Rizkalla AA, El-Assal S, Ali RT, Hussein MH. Effect of long-term heat stress on grain yield, pollen grain viability and germinability in bread wheat (Triticum aestivum L.) under field conditions. Heliyon. 2021;7(6): e07096.
Wu C, Cui K, Fahad S. Heat stress decreases rice grain weight: evidence and physiological mechanisms of heat effects prior to flowering. Int J Mol Sci. 2022;23(18):10922.
Roychowdhury R, Zilberman O, Chandrasekhar K, Curzon AY, Nashef K, Abbo S, Slafer GA, Bonfil DJ, Ben-David R. Pre-anthesis spike growth dynamics and its association to yield components among elite bread wheat cultivars (Triticum aestivum L. spp.) under Mediterranean climate. Field Crops Res. 2023;298: 108948.
Fernie E, Tan DKY, Liu SY, Ullah N, Khoddami A. Post-Anthesis heat influences grain yield, physical and nutritional quality in wheat: a review. Agriculture. 2022;12(6):886.
Kaushal N, Bhandari K, Siddique KH, Nayyar H. Food crops face rising temperatures: an overview of responses, adaptive mechanisms, and approaches to improve heat tolerance. Cogent Food Agric. 2016;2(1):1134380.
Schittenhelm S, Langkamp-Wedde T, Kraft M, Kottmann L, Matschiner K. Effect of two-week heat stress during grain filling on stem reserves, senescence, and grain yield of European winter wheat cultivars. J Agron Crop Sci. 2020;206(6):722–33.
Mason RE, Mondal S, Beecher FW, Pacheco A, Jampala B, Ibrahim AMH, et al. QTL associated with heat susceptibility index in wheat (Triticum aestivum L.) under short-term reproductive stage heat stress. Euphytica. 2010;174(3):423–36.
Hurkman WJ, Vensel WH, Tanaka CK, Whitehand L, Altenbach SB. Effect of high temperature on albumin and globulin accumulation in the endosperm proteome of the developing wheat grain. J Cereal Sci. 2009;49(1):12–23.
Suwa R, Hakata H, Hara H, El-Shemy HA, Adu-Gyamfi JJ, Nguyen NT, Kanai S, Lightfoot DA, Mohapatra PK, Fujita K. High temperature effects on photosynthate partitioning and sugar metabolism during ear expansion in maize (Zea mays L.) genotypes. Plant Physiol Biochem. 2010;48(2–3):124–30.
Djanaguiraman M, Prasad PVV, Al-Khatib K. Ethylene perception inhibitor 1-MCP decreases oxidative damage of leaves through enhanced antioxidant defense mechanisms in soybean plants grown under high temperature stress. Environ Exp Bot. 2011;71(2):215–23.
Rahman MA, Chikushi J, Yoshida S, Karim AJMS. Growth and yield components of wheat genotypes exposed to high temperature stress under control environment. Bangladesh J Agric Res. 2009;34(3):360–72.
Hasanuzzaman M, Nahar K, Fujita M. Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. Ecophysiol Responses Plants Under Salt Stress. 2013;25–87.
Djanaguiraman M, Prasad PV, Seppanen M. Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol Biochem. 2010;48(12):999–1007.
Saitoh H. Ecological and Physiology of Vegetable; Nousangyoson Bunka Kyoukai: Tokyo, Japan, 2008 (direct from paper reference)
Haque MS, Kjaer KH, Rosenqvist E, Sharma DK, Ottosen CO. Heat stress and recovery of photosystem II efficiency in wheat (Triticum aestivum L.) cultivars acclimated to different growth temperatures. Environ Exp Bot. 2014;99:1–8.
Iqbal M, Hussain I, Liaqat H, Ashraf MA, Rasheed R, Rehman AU. Exogenously applied selenium reduces oxidative stress and induces heat tolerance in spring wheat. Plant Physiol Biochem. 2015;94:95–103.
Yin Y, Li S, Liao W, Lu Q, Wen X, Lu C. Photosystem II photochemistry, photoinhibition, and the xanthophyll cycle in heat-stressed rice leaves. J Plant Physiol. 2010;167(12):959–66.
Zhang X, Cai J, Wollenweber B, Liu F, Dai T, Cao W, Jiang D. Multiple heat and drought events affect grain yield and accumulations of high molecular weight glutenin subunits and glutenin macropolymers in wheat. J Cereal Sci. 2013;57(1):134–40.
Yun-Ying C, Hua DUAN, Li-Nian YANG, Zhi-Qing WANG, Li-Jun LIU, Jian-Chang YANG. Effect of high temperature during heading and early filling on grain yield and physiological characteristics in indica rice. Acta Agron Sin. 2009;35(3):512–21.
Tan W, Wei Meng Q, Brestic M, Olsovska K, Yang X. Photosynthesis is improved by exogenous calcium in heat-stressed tobacco plants. J Plant Physiol. 2011;168(17):2063–71.
Edreira JIR, Otegui ME. Heat stress in temperate and tropical maize hybrids: differences in crop growth, biomass partitioning and reserves use. Field Crop Res. 2012;130:87–98.
Mohammed AR, Tarpley L. Effects of high night temperature and spikelet position on yield-related parameters of rice (Oryza sativa L.) plants. Eur J Agron. 2010;33(2):117–23.
Gunawardhana MDM, De Silva CS. Impact of temperature and water stress on growth yield and related biochemical parameters of okra. Trop Agric Res. 2012;23(1).
Faralli M, Lektemur C, Rosellini D, Gürel F. Effects of heat shock and salinity on barley growth and stress-related gene transcription. Biol Plant. 2015;59:537–46.
Scarano A, Olivieri F, Gerardi C, Liso M, Chiesa M, Chieppa M, Frusciante L, Barone A, Santino A, Rigano MM. Selection of tomato landraces with high fruit yield and nutritional quality under elevated temperatures. J Sci Food Agric. 2020;100(6):2791–9.
Alhaithloul HA, Galal FH, Seufi AM. Effect of extreme temperature changes on phenolic, flavonoid contents and antioxidant activity of tomato seedlings (Solanum lycopersicum L.). PeerJ. 2021;9: e11193.
Vijayakumar A, Shaji S, Beena R, Sarada S, Rani TS, Stephen R, Manju RV, Viji MM. High temperature induced changes in quality and yield parameters of tomato (Solanum lycopersicum L.) and similarity coefficients among genotypes using SSR markers. Heliyon. 2021;7(2): e05988.
Al-Huqail A, El-Dakak RM, Sanad MN, Badr RH, Ibrahim MM, Soliman D, Khan F. Effects of climate temperature and water stress on plant growth and accumulation of antioxidant compounds in sweet basil (Ocimum basilicum L.) leafy vegetable. Scientifica. 2020;2020(1):3808909.
Granado-Rodríguez S, Aparicio N, Matías J, Pérez-Romero LF, Maestro I, Gracés I, Pedroche JJ, Haros CM, Fernandez-Garcia N, Del Hierro JN, Martin D, Bolaños L, Reguera M. Studying the impact of different field environmental conditions on seed quality of quinoa: the case of three different years changing seed nutritional traits in southern Europe. Front Plant Sci. 2021;12: 649132.
Arrizabalaga-Arriazu M, Gomès E, Morales F, Irigoyen JJ, Pascual I, Hilbert G. High temperature and elevated carbon dioxide modify berry composition of different clones of grapevine (Vitis vinifera L.) cv Tempranillo. Front Plant Sci. 2020;11: 603687.
He J, Tan C, Qin L. Root-zone heat priming effects on maximum quantum efficiency of PSII, productivity, root morphology and nutritional quality of two aeroponically grown leafy greens in a tropical greenhouse. Plants. 2022;11(13):1684.
De Leonardis AM, Fragasso M, Beleggia R, Ficco DBM, De Vita P, Mastrangelo AM. Effects of heat stress on metabolite accumulation and composition, and nutritional properties of durum wheat grain. Int J Mol Sci. 2015;16(12):30382–404.
Collado-González J, Piñero MC, Otálora G, López-Marín J, Amor FMD. The effect of foliar putrescine application, ammonium exposure, and heat stress on antioxidant compounds in cauliflower waste. Antioxidants. 2021;10(5):707.
Tuteja N, Mahajan S. Calcium signaling network in plants: an overview. Plant Signal Behav. 2007;2(2):79–85. https://doi.org/10.4161/psb.2.2.4176.
Zheng Y, Wang X, Cui X, Wang K, Wang Y, He Y. Phytohormones regulate the abiotic stress: an overview of physiological, biochemical, and molecular responses in horticultural crops. Front Plant Sci. 2023;13:1095363.
Vishwakarma K, Upadhyay N, Kumar N, Yadav G, Singh J, Mishra RK, Kumar V, Verma R, Upadhyay RG, Pandey M, Sharma S. Abscisic acid signaling and abiotic stress tolerance in plants: a review on current knowledge and future prospects. Front Plant Sci. 2017. https://doi.org/10.3389/fpls.2017.00161.
Basu S, Rabara R. Abscisic acid—an enigma in the abiotic stress tolerance of crop plants. Plant Gene. 2017;11:90–8. https://doi.org/10.1016/j.plgene.2017.04.008.
Hu E, Liu M, Zhou R, Jiang F, Sun M, Wen J, Zhu Z, Wu Z. Relationship between melatonin and abscisic acid in response to salt stress of tomato. Sci Hortic. 2021;285: 110176. https://doi.org/10.1016/J.SCIENTA.2021.110176.
Awan KK, Awan FK, Khurshid MY, Mehmood A. Plant growth regulators and their role in abiotic stress management. Int J Innov Res Biosci. 2017;1:9–21.
Danquah A, de Zelicourt A, Colcombet J, Hirt H. The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol Adv. 2014;32(1):40–52. https://doi.org/10.1016/j.biotechadv.2013.09.006.
Yamaguchi-Shinozaki K, Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Ann Rev Plant Biol. 2006;57:781–803. https://doi.org/10.1146/annurev.arplant.57.032905.105444.
Xue-Xuan X, Hong-Bo S, Yuan-Yuan M, Gang X, Jun-Na S, Dong-Gang G, Cheng-Jiang R. Biotechnological implications from abscisic acid (ABA) roles in cold stress and leaf senescence as an important signal for improving plant sustainable survival under abiotic-stressed conditions. Crit Rev Biotechnol. 2010;30(3):222–30. https://doi.org/10.3109/07388551.2010.487186.
Zalabák D, Pospíšilová H, Šmehilová M, Mrízová K, Frébort I, Galuszka P. Genetic engineering of cytokinin metabolism: prospective way to improve agricultural traits of crop plants. Biotechnol Adv. 2013;31(1):97–117.
Masson-Delmotte VP, Zhai P, Pirani SL, Connors C, Péan S, Berger N, Scheel Monteiro PM. Ipcc, 2021: Summary for policymakers. in: Climate change 2021: The physical science basis. contribution of working group i to the sixth assessment report of the intergovernmental panel on climate change. 2021
Dobrá J, Černý M, Štorchová H, Dobrev P, Skalák J, Jedelský PL, Lukšanová H, Gaudinová A, Pešek B, Malbeck J, Vanek T, Brzobohatý B, Vanková R. The impact of heat stress targeting on the hormonal and transcriptomic response in Arabidopsis. Plant Sci. 2015;231:52–61.
Srivastava A, Pandey GC. Role of cytokinins and gibberellins in crops response to heat and drought stress. J Cereal Res. 2023;15(2):170–6.
Sobol S, Chayut N, Nave N, Kafle D, Hegele M, Kaminetsky R, Wünsche JN, Samach A. Genetic variation in yield under hot ambient temperatures spotlights a role for cytokinin in protection of developing floral primordia. Plant, Cell Environ. 2014;37(3):643–57.
Xing J, Xu Y, Tian J, Gianfagna T, Huang B. Suppression of shade-or heat-induced leaf senescence in creeping bentgrass through transformation with the ipt gene for cytokinin synthesis. J Am Soc Hortic Sci. 2009;134(6):602–9.
Skalák J, Černý M, Jedelský P, Dobrá J, Ge E, Novák J, Hronková M, Dobrev P, Vanková R, Brzobohatý B. Stimulation of ipt overexpression as a tool to elucidate the role of cytokinins in high temperature responses of Arabidopsis thaliana. J Exp Bot. 2016;67(9):2861–73.
Wu C, Cui K, Wang W, Li Q, Fahad S, Hu Q, Huang J, Nie L, Mohapatra PK, Peng S. Heat-induced cytokinin transportation and degradation are associated with reduced panicle cytokinin expression and fewer spikelets per panicle in rice. Front Plant Sci. 2017;8:371.
Yang D, Li Y, Shi Y, Cui Z, Luo Y, Zheng M, Chen J, Li Y, Yin Y, Wang Z. Exogenous cytokinins increase grain yield of winter wheat cultivars by improving stay-green characteristics under heat stress. PLoS ONE. 2016;11(5): e0155437.
Jespersen D, Yu J, Huang B. Metabolite responses to exogenous application of nitrogen, cytokinin, and ethylene inhibitors in relation to heat-induced senescence in creeping bentgrass. PLoS ONE. 2015;10(3): e0123744.
Mittler R, Finka A, Goloubinoff P. How do plants feel the heat? Trends Biochem Sci. 2012;37(3):118–25.
Zwack PJ, Rashotte AM. Interactions between cytokinin signalling and abiotic stress responses. J Exp Bot. 2015;66(16):4863–71.
Prerostova S, Dobrev PI, Kramna B, Gaudinova A, Knirsch V, Spichal L, Zatloukal M, Vankova R. Heat acclimation and inhibition of cytokinin degradation positively affect heat stress tolerance of Arabidopsis. Front Plant Sci. 2020;11:87.
Huber AE, Bauerle TL. Long-distance plant signaling pathways in response to multiple stressors: the gap in knowledge. J Exp Bot. 2016;67(7):2063–79.
Eyidogan F, Oz MT, Yucel M, Oktem HA. Signal transduction of phytohormones under abiotic stresses. Phytohormones Abiotic Stress Tolerance Plants. 2012; 1–48.
Ullah A, Sun H, Hakim, Yang X, Zhang X. A novel cotton WRKY gene, GhWRKY6-like, improves salt tolerance by activating the ABA signaling pathway and scavenging of reactive oxygen species. Physiol Plant. 2018;162(4):439–54.
Clarke SM, Cristescu SM, Miersch O, Harren FJ, Wasternack C, Mur LA. Jasmonates act with salicylic acid to confer basal thermotolerance in Arabidopsis thaliana. New Phytol. 2009;182(1):175–87.
Xu YH, Liao YC, Zhang Z, Liu J, Sun PW, Gao ZH, Sui C, Wei JH. Jasmonic acid is a crucial signal transducer in heat shock-induced sesquiterpene formation in Aquilaria sinensis. Sci Rep. 2016;6(1):21843.
Kumari S, Nazir F, Maheshwari C, Kaur H, Gupta R, Siddique KH, Khan MIR. Plant hormones and secondary metabolites under environmental stresses: shedding light on defense molecules. Plant Physiol Biochem. 2023;206: 108238.
Su Y, Huang Y, Dong X, Wang R, Tang M, Cai J, Chen J, Zhang X, Nie G. Exogenous methyl jasmonate improves heat tolerance of perennial ryegrass through alteration of osmotic adjustment, antioxidant defense, and expression of jasmonic acid-responsive genes. Front Plant Sci. 2021;12: 664519.
Swain R, Sahoo S, Behera M, Rout GR. Instigating prevalent abiotic stress resilience in crop by exogenous application of phytohormones and nutrient. Front Plant Sci. 2023;14:1104874.
Buttar ZA, Wu SN, Arnao MB, Wang C, Ullah I, Wang C. Melatonin suppressed the heat stress-induced damage in wheat seedlings by modulating the antioxidant machinery. Plants. 2020;9(7):809.
Tan DX, Hardeland R, Manchester LC, Poeggeler B, Lopez-Burillo S, Mayo JC, Sainz RM, Reiter RJ. Mechanistic and comparative studies of melatonin and classic antioxidants in terms of their interactions with the ABTS cation radical. J Pineal Res. 2003;34(4):249–59.
Reiter RJ, Paredes SD, Manchester LC, Tan DX. Reducing oxidative/nitrosative stress: a newly-discovered genre for melatonin. Crit Rev Biochem Mol Biol. 2009;44(4):175–200.
Arnao MB, Hernández-Ruiz J. Melatonin and its relationship to plant hormones. Ann Bot. 2018;121(2):195–207.
Arnao M, Hernández-Ruiz J. Role of melatonin to enhance phytoremediation capacity. Appl Sci. 2019;9(24):5293.
Liang C, Zheng G, Li W, Wang Y, Hu B, Wang H, Wu H, Qian Y, Zhu X-G, Tan D-X, Chen S-Y, Chu C. Melatonin delays leaf senescence and enhances salt stress tolerance in rice. J Pineal Res. 2015;59(1):91–101.
Ma X, Zhang J, Burgess P, Rossi S, Huang B. Interactive effects of melatonin and cytokinin on alleviating drought-induced leaf senescence in creeping bentgrass (Agrostis stolonifera). Environ Exp Bot. 2018;145:1–11.
Jahan MS, Guo S, Baloch AR, Sun J, Shu S, Wang Y, Ahammed GJ, Kabir K, Roy R. Melatonin alleviates nickel phytotoxicity by improving photosynthesis, secondary metabolism and oxidative stress tolerance in tomato seedlings. Ecotoxicol Environ Saf. 2020;197: 110593.
Tan XL, Fan ZQ, Kuang JF, Lu WJ, Reiter RJ, Lakshmanan P, Su X-G, Zhou J, Chen J-Y, Shan W. Melatonin delays leaf senescence of Chinese flowering cabbage by suppressing ABFs-mediated abscisic acid biosynthesis and chlorophyll degradation. J Pineal Res. 2019;67(1): e12570.
Shi X, Xu S, Mu D, Sadeghnezhad E, Li Q, Ma Z, Zhao L, Zhang Q, Wang L. Exogenous melatonin delays dark-induced grape leaf senescence by regulation of antioxidant system and senescence associated genes (SAGs). Plants. 2019;8(10):366.
Liang D, Shen Y, Ni Z, Wang Q, Lei Z, Xu N, Deng Q, Lin L, Wang J, Lv X, Xia H. Exogenous melatonin application delays senescence of kiwifruit leaves by regulating the antioxidant capacity and biosynthesis of flavonoids. Front Plant Sci. 2018;9:426.
Wang P, Sun X, Chang C, Feng F, Liang D, Cheng L, Ma F. Delay in leaf senescence of Malus hupehensis by long-term melatonin application is associated with its regulation of metabolic status and protein degradation. J Pineal Res. 2013;55(4):424–34.
Ahammed GJ, Xu W, Liu A, Chen S. Endogenous melatonin deficiency aggravates high temperature-induced oxidative stress in Solanum lycopersicum L. Environ Exp Bot. 2019;161:303–11.
Jia C, Yu X, Zhang M, Liu Z, Zou P, Ma J, Xu Y. Application of melatonin-enhanced tolerance to high-temperature stress in cherry radish (Raphanus sativus L. var. radculus pers). J Plant Growth Regul. 2020;39:631–40.
Kumar S, Yu R, Liu Y, Liu Y, Khan MN, Liu Y, Wang M, Zhu G. Exogenous melatonin enhances heat stress tolerance in sweetpotato by modulating antioxidant defense system, osmotic homeostasis and stomatal traits. Hortic Plant J. 2024. https://doi.org/10.1016/j.hpj.2023.12.006.
Hanci F, Çingi M, Akinci H. Influence of L-tryptophan and melatonin on germination of onion and leek seeds at different temperatures. Türkiye Tarımsal Araştırmalar Dergisi. 2019;6(2):214–21.
Zhao N, Sun Y, Wang D, Zheng J. Effects of exogenous melatonin on nitrogen metabolism in cucumber seedlings under high temperature stress. Plant Physiol Commun. 2012;48(6):557–64.
Liang D, Gao F, Ni Z, Lin L, Deng Q, Tang Y, Wang X, Luo X, Xia H. Melatonin improves heat tolerance in kiwifruit seedlings through promoting antioxidant enzymatic activity and glutathione S-transferase transcription. Molecules. 2018;23(3):584.
Pehlivan N, Guler NS. Protective effect of a natural ally on simultaneous mild heat and salt episodes in maize seedlings. Acta Physiol Plant. 2018;40(12):203.
Khan S, Alvi AF, Khan NA. Role of ethylene in the regulation of plant developmental processes. Stresses. 2024;4(1):28–53. https://doi.org/10.3390/stresses4010003.
Debnath B, Islam W, Li M, Sun Y, Lu X, Mitra S, Hussain M, Liu S, Qiu D. Melatonin mediates enhancement of stress tolerance in plants. Int J Mol Sci. 2019;20(5):1040.
Yin Z, Lu J, Meng S, Liu Y, Mostafa I, Qi M, Li T. Exogenous melatonin improves salt tolerance in tomato by regulating photosynthetic electron flux and the ascorbate–glutathione cycle. J Plant Interact. 2019;14(1):453–63.
Bagautdinova ZZ, Omelyanchuk N, Tyapkin AV, Kovrizhnykh VV, Lavrekha VV, Zemlyanskaya EV. Salicylic acid in root growth and development. Int J Mol Sci. 2022. https://doi.org/10.3390/ijms23042228.
Guo B, Liu C, Liang Y, Li N, Fu Q. Salicylic acid signals plant defence against cadmium toxicity. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20122960.
Khan MIR, Fatma M, Per TS, Anjum NA, Khan NA. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci. 2015. https://doi.org/10.3389/fpls.2015.00462.
Bandurska H, Stroiński A. The effect of salicylic acid on barley response to water deficit. Acta Physiol Plant. 2005;27(3):379–86. https://doi.org/10.1007/s11738-005-0015-5.
Liu HT, Liu YP, Huang WD. Root-fed salicylic acid in grape involves the response caused by aboveground high temperature. J Integr Plant Biol. 2008;50(6):761–7. https://doi.org/10.1111/j.1744-7909.2008.00640.x.
Xia XJ, Zhou YH, Shi K, Zhou J, Foyer CH, Yu JQ. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J Exp Bot. 2015;66(10):2839–56. https://doi.org/10.1093/jxb/erv089.
Tamás L, Mistrík I, Alemayehu A, Zelinová V, Bočová B, Huttová J. Salicylic acid alleviates cadmium-induced stress responses through the inhibition of Cd-induced auxin-mediated reactive oxygen species production in barley root tips. J Plant Physiol. 2015;173:1–8. https://doi.org/10.1016/j.jplph.2014.08.018.
Wang Q, Liang X, Dong Y, Xu L, Zhang X, Kong J, Liu S. Effects of exogenous salicylic acid and nitric oxide on physiological characteristics of perennial ryegrass under cadmium stress. J Plant Growth Regul. 2013;32(4):721–31. https://doi.org/10.1007/s00344-013-9339-3.
Raza A, Javed R, Zahid Z, Sharif R, Hafeez MB, Ghouri MZ, Nawaz MU, Siddiqui MH. Strigolactones for sustainable plant growth and production under adverse environmental conditions. Plant Performance Under Environmental Stress: Hormones, Biostimulants and Sustainable Plant Growth Management, 2021:129–166.
Banerjee A, Wani SH, Roychoudhury A. Epigenetic control of plant cold responses. Front Plant Sci. 2017;8: 301712.
Sytar O, Kumari P, Yadav S, Brestic M, Rastogi A. Phytohormone priming: regulator for heavy metal stress in plants. J Plant Growth Regul. 2019;38:739–52.
Saeed W, Naseem S, Ali Z. Strigolactones biosynthesis and their role in abiotic stress resilience in plants: a critical review. Front Plant Sci. 2017;8: 279971.
Banerjee A, Roychoudhury A. Strigolactones: multi-level regulation of biosynthesis and diverse responses in plant abiotic stresses. Acta Physiol Plant. 2018;40:1–10.
Omoarelojie LO, Kulkarni MG, Finnie JF, Pospíšil T, Strnad M, Van Staden J. Synthetic strigolactone (rac-GR24) alleviates the adverse effects of heat stress on seed germination and photosystem II function in lupine seedlings. Plant Physiol Biochem. 2020;155:965–79.
Zheng X, Li Y, Xi X, Ma C, Sun Z, Yang X, Li X, Tian Y, Wang C. Exogenous Strigolactones alleviate KCl stress by regulating photosynthesis, ROS migration and ion transport in Malus hupehensis Rehd. Plant Physiol Biochem. 2021;159:113–22.
Min Z, Li R, Chen L, Zhang Y, Li Z, Liu M, Ju Y, Fang Y. Alleviation of drought stress in grapevine by foliar-applied strigolactones. Plant Physiol Biochem. 2019;135:99–110.
Bhoi A, Yadu B, Chandra J, Keshavkant S. Contribution of strigolactone in plant physiology, hormonal interaction and abiotic stresses. Planta. 2021;254(2):28.
Koltai H, Beveridge CA. Strigolactones and the coordinated development of shoot and root. Long-distance systemic signaling and communication in plants, 2013:189–204.
Santoro V, Schiavon M, Gresta F, Ertani A, Cardinale F, Sturrock CJ, Celi L, Schubert A. Strigolactones control root system architecture and tip anatomy in Solanum lycopersicum L. plants under P starvation. Plants. 2020;9(5):612.
Brewer PB, Koltai H, Beveridge CA. Diverse roles of strigolactones in plant development. Mol Plant. 2013;6(1):18–28.
Jia KP, Luo Q, He SB, Lu XD, Yang HQ. Strigolactone-regulated hypocotyl elongation is dependent on cryptochrome and phytochrome signaling pathways in Arabidopsis. Mol Plant. 2014;7(3):528–40.
Camara MC, Vandenberghe LP, Rodrigues C, de Oliveira J, Faulds C, Bertrand E, Soccol CR. Current advances in gibberellic acid (GA 3) production, patented technologies, and potential applications. Planta. 2018;248:1049–62.
Yadav B, Jogawat A, Gnanasekaran P, Kumari P, Lakra N, Lal SK, Pawar J, Narayan OP. An overview of recent advancement in phytohormones-mediated stress management and drought tolerance in crop plants. Plant Gene. 2021;25: 100264.
Alexopoulos AA, Aivalakis G, Akoumianakis KA, Passam HC. Effect of foliar applications of gibberellic acid or daminozide on plant growth, tuberisation, and carbohydrate accumulation in tubers grown from true potato seed. J Hortic Sci Biotechnol. 2007;82(4):535–40.
Ahmed IU, Smith AR, Jones DL, Godbold DL. Tree species identity influences the vertical distribution of labile and recalcitrant carbon in a temperate deciduous forest soil. For Ecol Manage. 2016;359:352–60.
Gamel RE, Elsayed A, Bashasha J, Haroun S. Priming tomato cultivars in β-sitosterol or gibberellic acid improves tolerance for temperature stress. Int J Bot. 2017;13(1):1–14.
Wu C, Cui K, Wang W, Li Q, Fahad S, Hu Q, Huang J, Nie L, Peng S. Heat-induced phytohormone changes are associated with disrupted early reproductive development and reduced yield in rice. Sci Rep. 2016;6(1):34978.
Ahanger MA, Ashraf M, Bajguz A, Ahmad P. Brassinosteroids regulate growth in plants under stressful environments and crosstalk with other potential phytohormones. J Plant Growth Regul. 2018;37(4):1007–24. https://doi.org/10.1007/s00344-018-9855-2.
Nolan TM, Vukašinović N, Liu D, Russinova E, Yin Y. Brassinosteroids: multidimensional regulators of plant growth, development, and stress responses. Plant Cell. 2020;32(2):295–318. https://doi.org/10.1105/tpc.19.00335.
Fahad S, Nie L, Chen Y, Wu C, Xiong D, Saud S, Hongyan L, Cui K, Huang J. Crop plant hormones and environmental stress. Sustain Agric Rev: 2015;15:371–400. https://doi.org/10.1007/978-3-319-09132-7_10.
Manghwar H, Hussain A, Ali Q, Liu F. Brassinosteroids (BRs) role in plant development and coping with different stresses. Int J Mol Sci. 2022;23(3):1012. https://doi.org/10.3390/ijms23031012.
Janeczko A, Oklešťková J, Pociecha E, Kościelniak J, Mirek M. Physiological effects and transport of 24-epibrassinolide in heat-stressed barley. Acta Physiol Plant. 2011;33:1249–59. https://doi.org/10.1007/s11738-010-0655-y.
Wilen RW, Sacco M, Gusta LV, Krishna P. Effects of 24-epibrassinolide on freezing and thermotolerance of bromegrass (Bromus inermis) cell cultures. Physiol Plant. 1995;95(2):195–202. https://doi.org/10.1111/j.1399-3054.1995.tb00827.x.
Hayat SH, Mori M, Fariduddin QA, Bajguz AN, Ahmad A. Physiological role of brassinosteroids: an update. Indian J Plant Physiol. 2010;15:99–109.
Mazorra LM, Holton N, Bishop GJ, Núñez M. Heat shock response in tomato brassinosteroid mutants indicates that thermotolerance is independent of brassinosteroid homeostasis. Plant Physiol Biochem. 2011;49(12):1420–8. https://doi.org/10.1016/j.plaphy.2011.09.005.
Dhaubhadel S, Chaudhary S, Dobinson KF, Krishna P. Treatment with 24-epibrassinolide, a brassinosteroid, increases the basic thermotolerance of Brassica napus and tomato seedlings. Plant Mol Biol. 1999;40:333–42. https://doi.org/10.1023/A:1006283015582.
Thussagunpanit J, Jutamanee K, Sonjaroon W, Kaveeta L, Chai-Arree W, Pankean P, Suksamrarn A. Effects of brassinosteroid and brassinosteroid mimic on photosynthetic efficiency and rice yield under heat stress. Photosynthetica. 2015;53:312–20. https://doi.org/10.1007/s11099-015-0106-5.
Kaur H, Sirhindi G, Bhardwaj R, Alyemeni MN, Siddique KHM, Ahmad P. 28-homobrassinolide regulates antioxidant enzyme activities and gene expression in response to salt-and temperature-induced oxidative stress in Brassica juncea. Sci Rep. 2018;8(1):8735. https://doi.org/10.1038/s41598-018-27032-w.
González-Olmedo JL, Córdova A, Aragón CE, Pina D, Rivas M, Rodríguez R. Effect of an analogue of brassinosteroid on FHIA-18 plantlets exposed to thermal stress. 2005
Divi UK, Rahman T, Krishna P. Brassinosteroid-mediated stress tolerance in Arabidopsis shows interactions with abscisic acid, ethylene and salicylic acid pathways. BMC Plant Biol. 2010;10:1–14. https://doi.org/10.1186/1471-2229-10-151.
Anwar A, Liu Y, Dong R, Bai L, Yu X, Li Y. The physiological and molecular mechanism of brassinosteroid in response to stress: a review. Biol Res. 2018. https://doi.org/10.1186/s40659-018-0195-2.
Hussain M, Khan TA, Yusuf M, Fariduddin Q. Silicon-mediated role of 24-epibrassinolide in wheat under high-temperature stress. Environ Sci Pollut Res. 2019;26:17163–72.
Sakata T, Oshino T, Miura S, Tomabechi M, Tsunaga Y, Higashitani N, Miyazawa Y, Takahashi H, Watanabe M, Higashitani A. Auxins reverse plant male sterility caused by high temperatures. Proc Natl Acad Sci. 2010;107(19):8569–74.
Sharma L, Dalal M, Verma RK, Kumar SV, Yadav SK, Pushkar S, Kushwaha SR, Bhowmik A, Chinnusamy V. Auxin protects spikelet fertility and grain yield under drought and heat stresses in rice. Environ Exp Bot. 2018;150:9–24.
Abeysingha DN, Ozga JA, Strydhorst S, Doyle P, Iqbal M, Yang RC, Reinecke DM. The effect of auxins on amelioration of heat stress-induced wheat (Triticum aestivum L.) grain loss. J Agron Crop Sci. 2021;207(6):970–83.
Binder BM. Ethylene signaling in plants. J Biol Chem. 2020;295(22):7710–25. https://doi.org/10.1074/jbc.REV120.010854.
Chu P, Agapito-Tenfen SZ. Unintended genomic outcomes in current and next generation GM techniques: a systematic review. Plants. 2022. https://doi.org/10.3390/plants11212997.
Dubois M, Claeys H, Van den Broeck L, Inzé D. Time of day determines Arabidopsis transcriptome and growth dynamics under mild drought. Plant, Cell Environ. 2017;40(2):180–9. https://doi.org/10.1111/pce.12809.
McAtee P, Karim S, Schaffer R, David K. A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front Plant Sci. 2013. https://doi.org/10.3389/fpls.2013.00079.
Fatma M, Asgher M, Iqbal N, Rasheed F, Sehar Z, Sofo A, Khan NA. Ethylene signaling under stressful environments: analyzing collaborative knowledge. Plants. 2022. https://doi.org/10.3390/plants11172211.
Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, Maclean DJ, Ebert PR, Kazan K. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in arabidopsis. Plant Cell. 2004;16(12):3460–79. https://doi.org/10.1105/tpc.104.025833.
Steffens B. The role of ethylene and ROS in salinity, heavy metal, and flooding responses in rice. Front Plant Sci. 2014. https://doi.org/10.3389/fpls.2014.00685.
Liu M, Zhang H, Fang X, Zhang Y, Jin C. Auxin acts downstream of ethylene and nitric oxide to regulate magnesium deficiency-induced root hair development in Arabidopsis thaliana. Plant Cell Physiol. 2018;59(7):1452–65. https://doi.org/10.1093/pcp/pcy078.
Chen H, Bullock DA, Alonso JM, Stepanova AN. To fight or to grow: the balancing role of ethylene in plant abiotic stress responses. Plants. 2022. https://doi.org/10.3390/plants11010033.
Fei Q, Wei S, Zhou Z, Gao H, Li X. Adaptation of root growth to increased ambient temperature requires auxin and ethylene coordination in Arabidopsis. Plant Cell Rep. 2017;36(9):1507–18. https://doi.org/10.1007/s00299-017-2171-7.
Ramesh SA, Tyerman SD, Gilliham M, Xu B. γ-Aminobutyric acid (GABA) signalling in plants. Cell Mol Life Sci. 2017;74:1577–603.
Jespersen D, Zhang J, Huang B. Chlorophyll loss associated with heat-induced senescence in bentgrass. Plant Sci. 2016;249:1–12.
Ren T, Zheng P, Zhang K, Liao J, Xiong F, Shen Q, Ma Y, Fang W, Zhu X. Effects of GABA on the polyphenol accumulation and antioxidant activities in tea plants (Camellia sinensis L.) under heat-stress conditions. Plant Physiol Biochem. 2021;159:363–71.
Chen X, Li N, Liu C, Wang H, Li Y, Xie Y, Ma Y, Liang J, Li C. Exogenous GABA improves the resistance of apple seedlings to long-term drought stress by enhancing GABA shunt and secondary cell wall biosynthesis. Tree Physiol. 2022;42(12):2563–77.
Li Z, Yu J, Peng Y, Huang B. Metabolic pathways regulated by γ-aminobutyric acid (GABA) contributing to heat tolerance in creeping bentgrass (Agrostis stolonifera). Sci Rep. 2016;6(1):30338.
Nayyar H, Kaur R, Kaur S, Singh R. γ-Aminobutyric acid (GABA) imparts partial protection from heat stress injury to rice seedlings by improving leaf turgor and upregulating osmoprotectants and antioxidants. J Plant Growth Regul. 2014;33:408–19.
Zeng W, Hassan MJ, Kang D, Peng Y, Li Z. Photosynthetic maintenance and heat shock protein accumulation relating to γ-aminobutyric acid (GABA)-regulated heat tolerance in creeping bentgrass (Agrostis stolonifera). S Afr J Bot. 2021;141:405–13.
Li Z, Zhou M, Zeng W, Zhang Y, Liu L, Liu W, Peng Y. Root metabolites remodeling regulated by γ-aminobutyric acid (GABA) improves adaptability to high temperature in creeping bentgrass. Plant Soil. 2023;500:1–15.
Guo Z, Gong J, Luo S, Zuo Y, Shen Y. Role of gamma-aminobutyric acid in plant defense response. Metabolites. 2023;13(6):741.
Iqbal A, Dong Q, Wang X, Gui H, Zhang H, Zhang X, Song M. High nitrogen enhance drought tolerance in cotton through antioxidant enzymatic activities, nitrogen metabolism and osmotic adjustment. Plants. 2020;9(2):178.
Fois S, Motzo R, Giunta F. The effect of nitrogenous fertiliser application on leaf traits in durum wheat in relation to grain yield and development. Field Crop Res. 2009;110(1):69–75.
Gautam H, Sehar Z, Rehman MT, Hussain A, AlAjmi MF, Khan NA. Nitric oxide enhances photosynthetic nitrogen and sulfur-use efficiency and activity of ascorbate-glutathione cycle to reduce high temperature stress-induced oxidative stress in rice (Oryza sativa L.) plants. Biomolecules. 2021;11(2):305.
Ru C, Hu X, Chen D, Song T, Wang W, Lv M, Hansen NC. Nitrogen modulates the effects of short-term heat, drought and combined stresses after anthesis on photosynthesis, nitrogen metabolism, yield, and water and nitrogen use efficiency of wheat. Water. 2022;14(9):1407.
Nava G, Dechen AR, Nachtigall GR. Nitrogen and potassium fertilization affect apple fruit quality in southern Brazil. Commun Soil Sci Plant Anal. 2007;39(1–2):96–107.
Kumar V, Dwivedi P, Kumar P, Singh BN, Pandey DK, Kumar V, Bose B. Mitigation of heat stress responses in crops using nitrate primed seeds. S Afr J Bot. 2021;140:25–36.
Ge TD, Sun NB, Bai LP, Tong CL, Sui FG. Effects of drought stress on phosphorus and potassium uptake dynamics in summer maize (Zea mays) throughout the growth cycle. Acta Physiol Plant. 2012;34:2179–86.
Jiang M, Zhang J. Involvement of plasma-membrane NADPH oxidase in abscisic acid-and water stress-induced antioxidant defense in leaves of maize seedlings. Planta. 2002;215:1022–30.
Mphande W, Kettlewell PS, Grove IG, Farrell AD. The potential of antitranspirants in drought management of arable crops: a review. Agric Water Manag. 2020;236: 106143.
Niu C, Wang G, Sui J, Liu G, Ma F, Bao Z. Biostimulants alleviate temperature stress in tomato seedlings. Sci Hortic. 2022;293: 110712.
Hu J, Li Y, Jeong BR. Silicon alleviates temperature stresses in poinsettia by regulating stomata, photosynthesis, and oxidative damages. Agronomy. 2020;10(9):1419.
Shabbir R, Javed T, Hussain S, Ahmar S, Naz M, Zafar H, Pandey S, Chauhan J, Siddiqui MH, Pinghua C. Calcium homeostasis and potential roles in combatting environmental stresses in plants. S Afr J Bot. 2022;148:683–93.
Laanemets K, Brandt B, Li J, Merilo E, Wang YF, Keshwani MM, Taylor SS, Kollist H, Schroeder JI. Calcium-dependent and-independent stomatal signaling network and compensatory feedback control of stomatal opening via Ca2+ sensitivity priming. Plant Physiol. 2013;163(2):504–13.
Michal Johnson J, Reichelt M, Vadassery J, Gershenzon J, Oelmüller R. An Arabidopsis mutant impaired in intracellular calcium elevation is sensitive to biotic and abiotic stress. BMC Plant Biol. 2014;14:1–19.
Zeng H, Zhao B, Wu H, Zhu Y, Chen H. Comprehensive in silico characterization and expression profiling of nine gene families associated with calcium transport in soybean. Agronomy. 2020;10(10):1539.
Waraich EA, Ahmad R, Halim A, Aziz T. Alleviation of temperature stress by nutrient management in crop plants: a review. J Soil Sci Plant Nutr. 2012;12(2):221–44.
Cakmak I, Yazici AM. Magnesium: a forgotten element in crop production. Better crops. 2010;94(2):23–5.
Senbayram M, Gransee A, Wahle V, Thiel H. Role of magnesium fertilisers in agriculture: plant–soil continuum. Crop Pasture Sci. 2015;66(12):1219–29.
Mengutay M, Ceylan Y, Kutman UB, Cakmak I. Adequate magnesium nutrition mitigates adverse effects of heat stress on maize and wheat. Plant Soil. 2013;368:57–72.
Shahid M, Nayak AK, Tripathi R, Katara JL, Bihari P, Lal B, Gautam P. Boron application improves yield of rice cultivars under high temperature stress during vegetative and reproductive stages. Int J Biometeorol. 2018;62:1375–87.
Eisvand HR, Kamaei H, Nazarian F. Chlorophyll fluorescence, yield and yield components of bread wheat affected by phosphate bio-fertilizer, zinc and boron under late-season heat stress. Photosynthetica. 2018;56:1287–96.
Calderón-Páez SE, Cueto-Niño YA, Sánchez-Reinoso AD, Garces-Varon G, Chávez-Arias CC, Restrepo-Díaz H. Foliar boron compounds applications mitigate heat stress caused by high daytime temperatures in rice (Oryza sativa L.) Boron mitigates heat stress in rice. J Plant Nutr. 2021;44(17):2514–27.
Ullah A, Romdhane L, Rehman A, Farooq M. Adequate zinc nutrition improves the tolerance against drought and heat stresses in chickpea. Plant Physiol Biochem. 2019;143:11–8.
Han W, Huang L, Owojori OJ. Foliar application of zinc alleviates the heat stress of pakchoi (Brassica chinensis L.). J Plant Nutr. 2020;43(2):194–213.
Imtiaz M, Ashraf M, Rizwan MS, Nawaz MA, Rizwan M, Mehmood S, Yousaf B, Yuan Y, Ditta A, Mumtaz MA, Ali M, Mahmood S, Tu S. Vanadium toxicity in chickpea (Cicer arietinum L.) grown in red soil: effects on cell death, ROS and antioxidative systems. Ecotoxicol Environ Saf. 2018;158:139–44.
Tripathi DK, Vishwakarma K, Singh VP, Prakash V, Sharma S, Muneer S, Nikolic M, Deshmukh R, Vaculík M, Corpas FJ. Silicon crosstalk with reactive oxygen species, phytohormones and other signaling molecules. J Hazard Mater. 2021;408: 124820.
Chauhan R, Awasthi S, Srivastava S, Dwivedi S, Pilon-Smits EA, Dhankher OP, Tripathi RD. Understanding selenium metabolism in plants and its role as a beneficial element. Crit Rev Environ Sci Technol. 2019;49(21):1937–58.
Iqbal M, Shafiq F, Anwar S, Akram NA, Ashraf MA, Raza SH, Ali N, Ashraf M. Selenium and nano-selenium-mediated heat stress tolerance in plants. In Selenium and nano-selenium in environmental stress management and crop quality improvement. Cham: Springer International Publishing; 2022, 149–171
Mathur S, Sharma MP, Jajoo A. Improved photosynthetic efficacy of maize (Zea mays) plants with arbuscular mycorrhizal fungi (AMF) under high temperature stress. J Photochem Photobiol, B. 2018;180:149–54.
Seliem MK, Hafez Y, El-Ramady H. Using nano-selenium in reducing the negative effects of high temperature stress on Chrysanthemum morifolium ramat. J Sustain Agric Sci. 2020;46(3):47–60.
Leila B, El-Hafid N. Biofertilizers and biopesticides: Microbes for sustainable agriculture. Advances in plant microbiome and sustainable agriculture: Divers Biotechnol Appl. 2020:257–279.
Dordas C. Role of nutrients in controlling plant diseases in sustainable agriculture. A review. Agron Sustain Dev. 2008;28:33–46.
Basu S, Rabara RC, Negi S. AMF: the future prospect for sustainable agriculture. Physiol Mol Plant Pathol. 2018;102:36–45.
Srinivasarao CH. Programmes and policies for improving fertilizer use efficiency in agriculture. Indian J Fertil. 2021;17(3):226–54.
Mishra J, Prakash J, Kumar-Arora N. Role of beneficial soil microbes in sustainable agriculture and environmental management. Clim Chang Environ Sustain. 2016;4(2):137–49.
Rana KL, Kour D, Yadav AN. Endophytic microbiomes: biodiversity, ecological significance and biotechnological applications. Res J Biotechnol. 2019;14:142–62.
Ahmad P, Hashem A, Abd-Allah EF, Alqarawi AA, John R, Egamberdieva D, Gucel S. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L.) through antioxidative defense system. Front Plant Sci. 2015;6:868.
Yadav AN, Rastegari AA, Yadav N, Kour D Eds. Advances in plant microbiome and sustainable agriculture: diversity and biotechnological applications (Vol. 19). Springer Singapore. 2020. https://doi.org/10.1007/978-981-15-3208-5
Ali J, Mukarram M, Ojo J, Dawam N, Riyazuddin R, Ghramh HA, Khan KA, Chen R, Kurjak D, Bayram A. Harnessing phytohormones: advancing plant growth and defence strategies for sustainable agriculture. Physiol Plant. 2024;176(3): e14307.
Kosakivska IV, Vedenicheva NP, Babenko LM, Voytenko LV, Romanenko KO, Vasyuk VA. Exogenous phytohormones in the regulation of growth and development of cereals under abiotic stresses. Mol Biol Rep. 2022;49(1):617–28.
Fahad S, Hussain S, Saud S, Hassan S, Ihsan Z, Shah AN, Wu C, Yousaf M, Nasim W, Alharby H, Alghabari F, Huang J. Exogenously applied plant growth regulators enhance the morpho-physiological growth and yield of rice under high temperature. Front Plant Sci. 2016;7:1250.
Mukherjee A, Gaurav AK, Singh S, Yadav S, Bhowmick S, Abeysinghe S, Verma JP. The bioactive potential of phytohormones: a review. Biotechnol Rep. 2022;35: e00748.
Bottrell DG, Schoenly KG. Integrated pest management for resource-limited farmers: challenges for achieving ecological, social and economic sustainability. J Agric Sci. 2018;156(3):408–26.
Rahman SU, Li Y, Hussain S, Hussain B, Riaz L, Ashraf MN, Khaliq MA, Du Z, Cheng H. Role of phytohormones in heavy metal tolerance in plants: a review. Ecol Indicat. 2023;146: 109844.
Chávez-Dulanto PN, Thiry AA, Glorio-Paulet P, Vögler O, Carvalho FP. Increasing the impact of science and technology to provide more people with healthier and safer food. Food Energy Secur. 2021;10(1): e259.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
P.B.A. developed the idea and designed the structure. P.B.A., A.D., A.R.R., J.J.K., I.A., A.B., A.T.P., M.M. and S.T.Y. wrote the manuscript and prepared the final version of the manuscript. All authors read and agree to the submission of the manuscript to the journal.
Corresponding author
Ethics declarations
Consent for publication
The article contains no such material that may be unlawful, defamatory, or which would, if published, in any way whatsoever, violate the terms and conditions as laid down in the agreement.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Angon, P.B., Das, A., Roy, A.R. et al. Plant development and heat stress: role of exogenous nutrients and phytohormones in thermotolerance. Discov. Plants 1, 17 (2024). https://doi.org/10.1007/s44372-024-00020-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44372-024-00020-3