Abstract
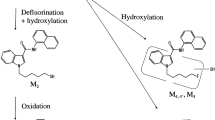
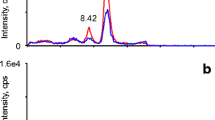
Two novel abiraterone (Abi, 3β-OH-Abi) metabolites in human serum were identified as 3α-OH-Abi and Δ5-Abi (D5A). Both metabolites were confirmed by their retention times on LC/MS and their product-ion mass spectra on LC–MS/MS compared to those of authentic compounds, which were chemically synthesized. The plausible metabolic pathways of these two metabolites are as follows: Abi is first oxidized to D5A by 3β-hydroxysteroid dehydrogenase (3β-HSD) and then irreversibly converted to Δ4-Abi (D4A) by ∆5–∆4 isomerase. Presumably, D5A detection is difficult because of its rapid conversion to D4A and its low concentration in serum samples. In contrast, the low concentration 3α-OH-Abi was generated by reducing the remaining D5A using 3α-hydroxysteroid dehydrogenase (3α-HSD).
Graphical Abstract











Similar content being viewed by others
Data availability
Not applicable.
References
G. Gandaglia, R. Leni, F. Bray, N. Fleshner, S.J. Freedland, A. Kibel, P. Stattin, H.V. Poppel, C. La Vecchia, Eur Urol Oncol 4, 877 (2021). https://doi.org/10.1016/j.euo.2021.09.006
J. Ferlay, M. Colombet, I. Soerjomataram, D.M. Parkin, M. Piñeros, A. Znaor, F. Bray, Int. J. Cancer 149, 778 (2021). https://doi.org/10.1002/ijc.33588
R.L. Siegel, K.D. Miller, H.E. Fuchs, A. Jemal, CA Cancer J. Clin. 72, 7 (2022). https://doi.org/10.3322/caac.21708
M.H. Tan, J. Li, H.E. Xu, K. Melcher, E.L. Yong, Acta Pharmacol. Sin. 36, 3 (2015). https://doi.org/10.1038/aps.2014.18
H. Charles, V.H. Clarence, Cancer Res. 1, 293 (1941)
Y. Li, S.C. Chan, L.J. Brand, T.H. Hwang, K.A.T. Silverstein, S.M. Dehm, Cancer Res. 73, 483 (2013). https://doi.org/10.1158/0008-5472.CAN-12-3630
S.S. Dutt, A.C. Gao, Future Oncol. 5, 1403 (2009). https://doi.org/10.2217/fon.09.117
A.H. Payne, D.B. Hales, Endocr. Rev. 25, 947 (2004). https://doi.org/10.1210/er.2003-0030
Z. Li, A.C. Bishop, M. Alyamani, J.A. Garcia, R. Dreicer, D. Bunch, J. Liu, S.K. Upadhyay, R.J. Auchus, N. Sharifi, Nature 523, 347 (2015). https://doi.org/10.1038/nature14406
Z. Li, M. Alyamani, J. Li, K. Rogacki, M. Abazeed, S.K. Upadhyay, S.P. Balk, M.E. Taplin, R.J. Auchus, N. Sharifi, Nature 533, 547 (2016). https://doi.org/10.1038/nature17954
H. Kanji, S. Horiyama, T. Kimachi, J. Haginaka, Anal. Sci. 37, 1281 (2021). https://doi.org/10.2116/analsci.21P035
M. Shiota, R. Inoue, K. Tashiro, K. Kobayashi, S. Horiyama, H. Kanji, M. Eto, S. Egawa, J. Haginaka, H. Matsuyama, J. Clin. Pharmacol. 37, 445 (2023). https://doi.org/10.1002/jcph.2191
J.L. Thomas, R.C. Strickler, R.P. Myers, D.F. Covey, Biochem. 31, 5522 (1992). https://doi.org/10.1021/bi00139a014
S.G. Cheatum, J.C. Warren, Biochim. Biophys. Acta 122, 1 (1966). https://doi.org/10.1016/0926-6593(66)90086-5
M.K. Rasmussen, B. Ekstrand, G. Zamaratskaia, Int. J. Mol. Sci. 14, 17926 (2013). https://doi.org/10.3390/ijms140917926
W.-N. Wu, L.A. McKown, Methods in pharmacology and toxicology optimization in drug discovery: in vitro methodsm (Humana Press, Totowa, 2004)
Acknowledgements
This study was partially supported by JSPS KAKENHI (Grant no. 22K06571). We thank Dr. Taro Sakamoto of Bruker Japan for his help regarding MS/MS.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Horiyama, S., Hayama, N., Yoneyama, H. et al. Identification of novel metabolites of abiraterone in human serum and their metabolic pathways. ANAL. SCI. 40, 67–74 (2024). https://doi.org/10.1007/s44211-023-00431-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44211-023-00431-4