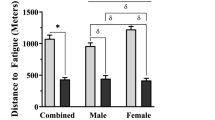
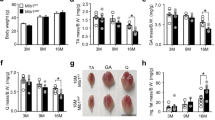
Abstract
Autophagy, a highly conserved quality control mechanism, is essential for the maintenance of cellular homeostasis and for the orchestration of an efficient cellular response to stress. During aging, the efficiency of autophagic degradation declines, and intracellular waste products accumulate. Therefore, in this study, we tested the hypothesis that skeletal muscle from old mice would have decreased autophagosome formation when compared to the muscle from young mice. We also examined whether autophagic regulatory events differ between muscle fiber types and in response to exercise in aged male mice. The extensor digitorum longus (EDL) and gastrocnemius muscles were studied in young and old ICR mice. Exercise was performed by allowing the mice to run on a treadmill with a 5° incline at 16.4 m/min for 40 min/day, 5 days/week for 8 weeks after a 1-week adaptation period. Our results indicated that the levels of microtubule-associated protein 1b light chain 3, a marker of autophagosome formation, were lower in both the EDL and the gastrocnemius muscle of old mice compared to those young mice. To identify the factors related to the changes observed, the expression of autophagy regulatory proteins was examined in the EDL and gastrocnemius muscles. Beclin-1, autophagy-related gene 7 (ATG7), and lysosome-associated membrane protein were found to be lower in the EDL and gastrocnemius muscles of old mice compared to those in the young mice, then Beclin-1, ATG7, and muscle-specific RING finger protein-1 upregulated after regular exercise. Moreover, the muscle weight/body weight was significantly increased only in the gastrocnemius muscle of the old trained mice. These data suggest that autophagy regulatory events are attenuated in old skeletal muscle. However, this effect is upregulated when animals are subjected to exercise training.





Similar content being viewed by others
References
Attaix D, Baracos VE (2010) MAFbx/Atrogin-1 expression is a poor index of muscle proteolysis. Curr Opin Clin Nutr Metab Care 13:223–224
Barth S, Glick D, Macleod KF (2010) Autophagy: assays and artifacts. J Pathol 221:117–124
Cao Y, Klionsky DJ (2007) Physiological functions of Atg6/Beclin 1: a unique autophagy-related protein. Cell Res 17:839–849
Cuervo AM (2008) Autophagy and aging: keeping that old broom working. Trends Genet 24:604–612
Cuervo AM, Dice JF (2000) Regulation of LAMP2a levels in the lysosomal membrane. Traffic 1:570–583
Edstrom E, Altun M, Hagglund M, Ulfhake B (2006) Atrogin-1/MAFbx and MuRF1 are downregulated in aging-related loss of skeletal muscle. J Gerontol A Biol Sci Med Sci 61:663–674
Eskelinen EL, Saftig P (2009) Autophagy: a lysosomal degradation pathway with a central role in health and disease. Biochim Biophys Acta 1793:664–673
Glass DJ (2005) Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol 37:1974–1984
He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, Korsmeyer S, Packer M, May HI, Hill JA, Virgin HW, Gilpin C, Xiao G, Bassel-Duby R, Scherer PE, Levine B (2012) Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature 481:511–515
Kim YA, Kim YS, Song W (2012) Autophagic response to a single bout of moderate exercise in murine skeletal muscle. J Physiol Biochem 68:229–235
Koyama S, Hata S, Witt CC, Ono Y, Lerche S, Ojima K, Chiba T, Doi N, Kitamura F, Tanaka K, Abe K, Witt SH, Rybin V, Gasch A, Franz T, Labeit S, Sorimachi H (2008) Muscle RING-finger protein-1 (MuRF1) as a connector of muscle energy metabolism and protein synthesis. J Mol Biol 376:1224–1236
Leger B, Cartoni R, Praz M, Lamon S, Deriaz O, Crettenand A, Gobelet C, Rohmer P, Konzelmann M, Luthi F, Russell AP (2006) Akt signalling through GSK-3beta, mTOR and Foxo1 is involved in human skeletal muscle hypertrophy and atrophy. J Physiol 576:923–933
Maiuri MC, Zalckvar E, Kimchi A, Kroemer G (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8:741–752
Marzetti E, Hwang JC, Lees HA, Wohlgemuth SE, Dupont-Versteegden EE, Carter CS, Bernabei R, Leeuwenburgh C (2010) Mitochondrial death effectors: relevance to sarcopenia and disuse muscle atrophy. Biochim Biophys Acta 1800:235–244
Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, Metzger D, Reggiani C, Schiaffino S, Sandri M (2009) Autophagy is required to maintain muscle mass. Cell Metab 10:507–515
Masiero E, Sandri M (2010) Autophagy inhibition induces atrophy and myopathy in adult skeletal muscles. Autophagy 6:307–309
Massey AC, Kaushik S, Sovak G, Kiffin R, Cuervo AM (2006) Consequences of the selective blockage of chaperone-mediated autophagy. Proc Natl Acad Sci USA 103:5805–5810
McElhinny AS, Perry CN, Witt CC, Labeit S, Gregorio CC (2004) Muscle-specific RING finger-2 (MURF-2) is important for microtubule, intermediate filament and sarcomeric M-line maintenance in striated muscle development. J Cell Sci 117:3175–3188
McMullen CA, Ferry AL, Gamboa JL, Andrade FH, Dupont-Versteegden EE (2009) Age-related changes of cell death pathways in rat extraocular muscle. Exp Gerontol 44:420–425
Melendez A, Talloczy Z, Seaman M, Eskelinen EL, Hall DH, Levine B (2003) Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science 301:1387–1391
Mizushima N (2009) Physiological functions of autophagy. Curr Top Microbiol Immunol 335:71–84
Ogata T, Oishi Y, Higuchi M, Muraoka I (2010) Fasting-related autophagic response in slow- and fast-twitch skeletal muscle. Biochem Biophys Res Commun 394:136–140
Rajawat YS, Hilioti Z, Bossis I (2009) Aging: central role for autophagy and the lysosomal degradative system. Ageing Res Rev 8:199–213
Salminen A, Kaarniranta K (2009) Regulation of the aging process by autophagy. Trends Mol Med 15:217–224
Salminen A, Kaarniranta K (2009) SIRT1: regulation of longevity via autophagy. Cell Signal 21:1356–1360
Sandri M (2008) Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 23:160–170
Sandri M (2010) Autophagy in health and disease. 3. Involvement of autophagy in muscle atrophy. Am J Physiol Cell Physiol 298:C1291–1297
Sandri M (2010) Autophagy in skeletal muscle. FEBS Lett 584:1411–1416
Simonsen A, Cumming RC, Brech A, Isakson P, Schubert DR, Finley KD (2008) Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy 4:176–184
Terman A, Gustafsson B, Brunk UT (2007) Autophagy, organelles and ageing. J Pathol 211:134–143
Toth ML, Sigmond T, Borsos E, Barna J, Erdelyi P, Takacs-Vellai K, Orosz L, Kovacs AL, Csikos G, Sass M, Vellai T (2008) Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Autophagy 4:330–338
Witt CC, Witt SH, Lerche S, Labeit D, Back W, Labeit S (2008) Cooperative control of striated muscle mass and metabolism by MuRF1 and MuRF2. EMBO J 27:350–360
Wohlgemuth SE, Julian D, Akin DE, Fried J, Toscano K, Leeuwenburgh C, Dunn WA Jr (2007) Autophagy in the heart and liver during normal aging and calorie restriction. Rejuvenation Res 10:281–292
Wohlgemuth SE, Seo AY, Marzetti E, Lees HA, Leeuwenburgh C (2010) Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life-long exercise. Exp Gerontol 45:138–148
Yen WL, Klionsky DJ (2008) How to live long and prosper: autophagy, mitochondria, and aging. Physiology (Bethesda) 23:248–262
Acknowledgments
Our work is supported by the Basic Science Research Program through the National Research Foundation of Korea, which is funded by the Ministry of Education, Science and Technology (2010-0015964) and was also partly supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MEST) (2011-0030133).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, Y.A., Kim, Y.S., Oh, S.L. et al. Autophagic response to exercise training in skeletal muscle with age. J Physiol Biochem 69, 697–705 (2013). https://doi.org/10.1007/s13105-013-0246-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-013-0246-7