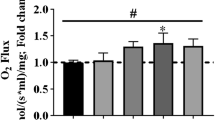
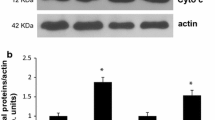
Abstract
The effect of a single bout of exercise on autopahgy in murine gastrocnemius muscle was investigated. Autophagy is a process for the degradation system of cytoplasmic components, which may help maintain intracellular quality control of cell survival and turnover under normal conditions. The present study investigated the changes of autophagy-related proteins including microtubule-associated protein 1b light chain 3 (LC3), Beclin-1, Atg7 (autophagy-related gene 7), conjugation form of Atg12 to Atg5, lysosome-associated membrane protein (LAMP2a), and muscle-specific RING finger protein-1 (MURF-1) protein level in gastrocnemius muscle after a single bout of treadmill exercise. Mice exercised on a treadmill for 50 min at a speed of 12.3 m/min with a slope of 5°. The animals were sacrificed by cervical dislocation 0, 3, 6, or 12 h after exercise, and muscle samples were collected immediately. Western blot analysis demonstrated that the autophagy marker LC3-II was significantly decreased during the recovery period (3, 6, and 12 h) whereas there was no decrease immediately after exercise (0 h). To identify factors related to this decrease, autophagosome component proteins were examined in murine gastrocnemius muscle. A decrease in Beclin-1, Atg7, and LAMP2a during recovery period was concomitant with the decreased level of LC3-II. Additionally, MuRF-1 expression was significantly increased after a single bout of exercise. This study is the first to demonstrate autophasic related protein expression after a single bout of treadmill exercise and our results suggest that a single bout of treadmill exercise attenuates the autophagic response in murine skeletal muscle.




Similar content being viewed by others
References
Barth S, Glick D, Macleod KF (2010) Autophagy: assays and artifacts. J Pathol 221:117–124
Cao Y, Klionsky DJ (2007) Physiological functions of Atg6/Beclin 1: a unique autophagy-related protein. Cell Res 17:839–849
Cuervo AM (2008) Autophagy and aging: keeping that old broom working. Trends Genet 24:604–612
Eskelinen EL, Saftig P (2009) Autophagy: a lysosomal degradation pathway with a central role in health and disease. Biochim Biophys Acta 1793:664–673
Maiuri MC, Zalckvar E, Kimchi A, Kroemer G (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8:741–752
Masiero E, Sandri M (2010) Autophagy inhibition induces atrophy and myopathy in adult skeletal muscles. Autophagy 6:307–309
Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, Metzger D, Reggiani C, Schiaffino S, Sandri M (2009) Autophagy is required to maintain muscle mass. Cell Metab 10:507–515
McElhinny AS, Perry CN, Witt CC, Labeit S, Gregorio CC (2004) Muscle-specific RING finger-2 (MURF-2) is important for microtubule, intermediate filament and sarcomeric M-line maintenance in striated muscle development. J Cell Sci 117:3175–3188
McMullen CA, Ferry AL, Gamboa JL, Andrade FH, Dupont-Versteegden EE (2009) Age-related changes of cell death pathways in rat extraocular muscle. Exp Gerontol 44:420–425
Mizushima N (2009) Physiological functions of autophagy. Curr Top Microbiol Immunol 335:71–84
Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y (2004) In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell 15:1101–1111
Raben N, Hill V, Shea L, Takikita S, Baum R, Mizushima N, Ralston E, Plotz P (2008) Suppression of autophagy in skeletal muscle uncovers the accumulation of ubiquitinated proteins and their potential role in muscle damage in Pompe disease. Hum Mol Genet 17:3897–3908
Rajawat YS, Hilioti Z, Bossis I (2009) Aging: central role for autophagy and the lysosomal degradative system. Ageing Res Rev 8:199–213
Salminen A, Kaarniranta K (2009) Regulation of the aging process by autophagy. Trends Mol Med 15:217–224
Salminen A, Kaarniranta K (2009) SIRT1: regulation of longevity via autophagy. Cell Signal 21:1356–1360
Sandri M (2010) Autophagy in skeletal muscle. FEBS Lett 584:1411–1416
Vabulas RM, Hartl FU (2005) Protein synthesis upon acute nutrient restriction relies on proteasome function. Science 310:1960–1963
Wang J (2008) Beclin 1 bridges autophagy, apoptosis and differentiation. Autophagy 4:947–948
Wohlgemuth SE, Julian D, Akin DE, Fried J, Toscano K, Leeuwenburgh C, Dunn WA Jr (2007) Autophagy in the heart and liver during normal aging and calorie restriction. Rejuvenation Res 10:281–292
Wohlgemuth SE, Seo AY, Marzetti E, Lees HA, Leeuwenburgh C (2010) Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life-long exercise. Exp Gerontol 45:138–148
Yen WL, Klionsky DJ (2008) How to live long and prosper: autophagy, mitochondria, and aging. Physiology (Bethesda) 23:248–262
Acknowledgments
Our work is supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2010-0015964) and NRF-2010-0009915.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, Y.A., Kim, Y.S. & Song, W. Autophagic response to a single bout of moderate exercise in murine skeletal muscle. J Physiol Biochem 68, 229–235 (2012). https://doi.org/10.1007/s13105-011-0135-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-011-0135-x