Abstract
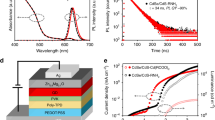
The CdSe/CdS/ZnS core/shell/shell quantum dots (QDs) with strong exciton confinement have manifested themselves as competitive light-emitting materials in electrochemiluminescence (ECL). However, cathodic ECL generation by these QDs requires the injection of electron and hole from solid electrode and electrogenerated radicals (for example SO4•−), which is inevitably influenced by not only the inorganic structure of QDs but also the organic ligands on the surface. In this work we aimed at studying the impact of surface organic ligands on ECL performance of CdSe/CdS/ZnS QDs. When changing the surface ligand from oleate to acetate, we phenomenologically observed the positive shift of ECL onset potential by ca. 200 mV and the increase of ECL intensity by ∼ 100 times, suggesting that a short ligand is more favorable for ECL generation. To further comprehend the ligand effect, we measured the charge injection kinetics using potential-modulated, time-resolved photoluminescence, and thin-layer spectroelectrochemistry techniques. The electron and hole injection into QDs were found to be accelerated by 2–20 times if shortening the ligand from oleate to acetate, confirming the significant impact of surface ligands on ECL performance of QDs. The study is expected to provide guidance on how to design surface functionalized QDs for specific applications such as ECL immunodiagnosis, photocatalysis, and photovoltaics.

Similar content being viewed by others
References
Steigerwald, M. L.; Brus, L. E. Semiconductor crystallites: A class of large molecules. Acc. Chem. Res. 1990, 23, 183–188.
Alivisatos, A. P. Semiconductor clusters, nanocrystals, and quantum dots. Science 1996, 277, 933–937.
Shu, Y. F.; Lin, X.; Qin, H. Y.; Hu, Z.; Jin, Y. Z.; Peng, X. G. Quantum dots for display applications. Angew. Chem., Int. Ed. 2020, 732, 22496–22507.
Colvin, V. L.; Schlamp, M. C.; Alivisatos, A. P. Light-emitting diodes made from cadmium selenide nanocrystals and a semiconducting polymer. Nature 1994, 370, 354–357.
Kwak, J.; Bae, W. K.; Lee, D.; Park, I.; Lim, J.; Park, M.; Cho, H.; Woo, H.; Yoon, D. Y.; Char, K. et al. Bright and efficient full-color colloidal quantum dot light-emitting diodes using an inverted device structure. Nano Lett. 2012, 12, 2362–2366.
Mashford, B. S.; Stevenson, M.; Popovic, Z.; Hamilton, C.; Zhou, Z. Q.; Breen, C.; Steckel, J.; Bulovic, V.; Bawendi, M.; Coe-Sullivan, S. et al. High- efficiency quantum-dot light-emitting devices with enhanced charge injection. Nat. Photon. 2013, 7, 407–412.
Dai, X. L.; Zhang, Z. X.; Jin, Y. Z.; Niu, Y.; Cao, H. J.; Liang, X. Y.; Chen, L. W.; Wang, J. P.; Peng, X. G. Solution-processed, highperformance light-emitting diodes based on quantum dots. Nature 2014, 515, 96–99.
Yang, Z. W.; Gao, M. Y.; Wu, W. J.; Yang, X. Y.; Sun, X. W.; Zhang, J. H.; Wang, H. C.; Liu, R. S.; Han, C. Y.; Yang, H. E. S. et al. Recent advances in quantum dot-based light-emitting devices: Challenges and possible solutions. Mater. Today 2019, 24, 69–93.
Ipe, B. I.; Lehnig, M.; Niemeyer, C. M. On the generation of free radical species from quantum dots. Small 2005, 1, 706–709.
Han, Z. J.; Qiu, F.; Eisenberg, R.; Holland, P. L.; Krauss, T. D. Robust photogeneration of H2 in water using semiconductor nanocrystals and a nickel catalyst. Science 2012, 338, 1321–1324.
Wu, H. L.; Li, X. B.; Tung, C. H.; Wu, L. Z. Semiconductor quantum dots: An emerging candidate for CO2 photoreduction. Adv. Mater. 2019, 31, 1900709.
Nozik, A. J.; Beard, M. C.; Luther, J. M.; Law, M.; Ellingson, R. J.; Johnson, J. C. Semiconductor quantum dots and quantum dot arrays and applications of multiple exciton generation to third-generation photovoltaic solar cells. Chem. Rev. 2010, 110, 6873–6890.
Semonin, O. E.; Luther, J. M.; Beard, M. C. Quantum dots for next-generation photovoltaics. Mater. Today 2012, 15, 508–515.
Duan, L. P.; Hu, L.; Guan, X. W.; Lin, C. H.; Chu, D. W.; Huang, S. J.; Liu, X. G.; Yuan, J. Y.; Wu, T. Quantum dots for photovoltaics: A tale of two materials. Adv. Energy Mater. 2021, 11, 2100354.
Dubertret, B.; Skourides, P.; Norris, D. J.; Noireaux, V.; Brivanlou, A. H.; Libchaber, A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science 2002, 298, 1759–1762
Gao, X. H.; Cui, Y. Y.; Levenson, R. M.; Chung, L. W. K.; Nie, S. M. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004, 22, 969–976
Michalet, X.; Pinaud, F. F.; Bentolila, L. A.; Tsay, J. M.; Doose, S.; Li, J. J.; Sundaresan, G.; Wu, A. M.; Gambhir, S. S.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544.
Wolfbeis, O. S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768.
Zhang, C. Y.; Yeh, H. C.; Kuroki, M. T.; Wang, T. H. Single-quantum-dot-based DNA nanosensor. Nat. Mater. 2005, 4, 826–831.
Wang, J.; Jiang, C. X.; Jin, J. N.; Huang, L.; Yu, W. B.; Su, B.; Hu, J. Ratiometric fluorescent lateral flow immunoassay for point-of-care testing of acute myocardial infarction. Angew. Chem., Int. Ed. 2021, 133, 13152–13159.
Martins, C. S. M.; LaGrow, A. P.; Prior, J. A. V. Quantum dots for cancer-related miRNA monitoring. ACS Sens. 2022, 7, 1269–1299.
Wu, P.; Hou, X. D.; Xu, J. J.; Chen, H. Y. Electrochemically generated versus photoexcited luminescence from semiconductor nanomaterials: Bridging the valley between two worlds. Chem. Rev. 2014, 114, 11027–11059.
Guo, W. L.; Ding, H.; Gu, C. Y.; Liu, Y. H.; Jiang, X. C.; Su, B.; Shao, Y. H. Potential-resolved multicolor electrochemiluminescence for multiplex immunoassay in a single sample. J. Am. Chem. Soc. 2018, 140, 15904–15915.
Zhao, Y. R.; Bouffier, L.; Xu, G. B.; Loget, G.; Sojic, N. Electrochemiluminescence with semiconductor (nano)materials. Chem. Sci. 2022, 73, 2528–2550.
Wang, Y. F.; Ding, J. L.; Zhou, P.; Liu, J. L.; Qiao, Z. Y.; Yu, K.; Jiang, J.; Su, B. Electrochemiluminescence distance and reactivity of coreactants determine the sensitivity of bead-based immunoassays. Angew. Chem., Int. Ed. 2023, 62, e202216525.
Yang, X. R.; Hang, J. M.; Qu, W. Y.; Wang, Y. L.; Wang, L.; Zhou, P.; Ding, H.; Su, B.; Lei, J.; Guo, W. L. et al. Gold microbeads enabled proximity electrochemiluminescence for highly sensitive and size-encoded multiplex immunoassays. J. Am. Chem. Soc. 2023, 145, 16026–16036.
Ding, Z. F.; Quinn, B. M.; Haram, S. K.; Pell, L. E.; Korgel, B. A.; Bard, A. J. Electrochemistry and electrogenerated chemiluminescence from silicon nanocrystal quantum dots. Science 2002, 296, 1293–1297.
Myung, N.; Ding, Z. F.; Bard, A. J. Electrogenerated chemiluminescence of CdSe nanocrystals. Nano Lett. 2002, 2, 1315–1319.
Myung, N.; Bae, Y.; Bard, A. J. Effect of surface passivation on the electrogenerated chemiluminescence of CdSe/ZnSe nanocrystals. Nano Lett. 2003, 3, 1053–1055.
Bae, Y.; Myung, N.; Bard, A. J. Electrochemistry and electrogenerated chemiluminescence of CdTe nanoparticles. Nano Lett. 2004, 4, 1153–1161.
Sun, L. F.; Bao, L.; Hyun, B. R.; Bartnik, A. C.; Zhong, Y. W.; Reed, J. C.; Pang, D. W.; Abruña, H. D.; Malliaras, G. G.; Wise, F. W. Electrogenerated chemiluminescence from PbS quantum dots. Nano Lett. 2009, 9, 789–793.
Liang, G. X.; Li, L. L.; Liu, H. Y.; Zhang, J. R.; Burda, C.; Zhu, J. J. Fabrication of near-infrared-emitting CdSeTe/ZnS core/shell quantum dots and their electrogenerated chemiluminescence. Chem. Commun. 2010, 46, 2974–2976.
Cao, Z. Y.; Shu, Y. F.; Qin, H. Y.; Su, B.; Peng, X. G. Quantum dots with highly efficient, stable, and multicolor electrochemiluminescence. ACS Cent. Sci. 2020, 6, 1129–1137.
Cao, Z. Y.; Li, C. Y.; Shu, Y. F.; Zhu, M. Y.; Su, B.; Qin, H. Y.; Peng, X. G. Unraveling mechanisms of highly efficient yet stable electrochemiluminescence from quantum dots. J. Am. Chem. Soc. 2023, 145, 26425–26434.
Ding, J. L.; Zhou, P.; Su, B. Quantum efficiency of electrochemiluminescence generation by tris(2,2′- bipyridine)ruthenium(II) and tri-n-propylamine revisited from a kinetic reaction model. ChemElectroChem 2022, 9, e202200236.
Tagliazucchi, M.; Tice, D. B.; Sweeney, C. M.; Morris-Cohen, A. J.; Weiss, E. A. Ligand- controlled rates of photoinduced electron transfer in hybrid CdSe nanocrystal/poly(viologen) films. ACS Nano 2011, 5, 9907–9917.
Ding, T. X.; Olshansky, J. H.; Leone, S. R.; Alivisatos, A. P. Efficiency of hole transfer from photoexcited quantum dots to covalently linked molecular species. J. Am. Chem. Soc. 2015, 137, 2021–2029.
Rosen, E. L.; Buonsanti, R.; Llordes, A.; Sawvel, A. M.; Milliron, D. J.; Helms, B. A. Exceptionally mild reactive stripping of native ligands from nanocrystal surfaces by using meerwein’s salt. Angew. Chem., Int. Ed. 2012, 51, 684–689.
Chang, C. M.; Orchard, K. L.; Martindale, B. C. M.; Reisner, E. Ligand removal from CdS quantum dots for enhanced photocatalytic H2 generation in pH neutral water. J. Mater. Chem.A 2016, 4, 2856–2862.
Bernt, C. M.; Burks, P. T.; DeMartino, A. W.; Pierri, A. E.; Levy, E. S.; Zigler, D. F.; Ford, P. C. Photocatalytic carbon disulfide production via charge transfer quenching of quantum dots. J. Am. Chem. Soc. 2014, 136, 2192–2195.
Wang, W. T.; Kapur, A.; Ji, X.; Safi, M.; Palui, G.; Palomo, V.; Dawson, P. E.; Mattoussi, H. Photoligation of an amphiphilic polymer with mixed coordination provides compact and reactive quantum dots. J. Am. Chem. Soc. 2015, 137, 5438–5451.
He, C.; Weinberg, D. J.; Nepomnyashchii, A. B.; Lian, S. C.; Weiss, E. A. Control of the redox activity of PbS quantum dots by tuning electrostatic interactions at the quantum dot/solvent interface. J. Am. Chem. Soc. 2016, 138, 8847–8854.
He, C.; Nguyen, T. D.; Edme, K.; De La Cruz, M. O.; Weiss, E. A. Noncovalent control of the electrostatic potential of quantum dots through the formation of interfacial ion pairs. J. Am. Chem. Soc. 2017, 139, 10126–10132.
Zhang, Z. Y.; Edme, K.; Lian, S. C.; Weiss, E. A. Enhancing the rate of quantum-dot-photocatalyzed carbon-carbon coupling by tuning the composition of the dot’s ligand shell. J. Am. Chem. Soc. 2017, 139, 4246–4249.
Vokhmintcev, K. V.; Samokhvalov, P. S.; Nabiev, I. Charge transfer and separation in photoexcited quantum dot-based systems. Nano Today 2016, 11, 189–211.
Winkler, J. R.; Gray, H. B. Long- range electron tunneling. J. Am. Chem. Soc. 2014, 136, 2930–2939.
Xu, W. W.; Hou, X. Q.; Meng, Y. J.; Meng, R. Y.; Wang, Z. Y.; Qin, H. Y.; Peng, X. G.; Chen, X. W. Deciphering charging status, absolute quantum efficiency, and absorption cross section of multicarrier states in single colloidal quantum dots. Nano Lett. 2017, 17, 7487–7493.
Jha, P. P.; Guyot-Sionnest, P. Trion decay in colloidal quantum dots. ACS Nano 2009, 3, 1011–1015.
Franceschetti, A.; Zunger, A. Optical transitions in charged CdSe quantum dots. Phys. Rev. B 2000, 62, R16287–R16290.
Hu, Z.; Liu, S. J.; Qin, H. Y.; Zhou, J. H.; Peng, X. G. Oxygen stabilizes photoluminescence of CdSe/CdS core/shell quantum dots via deionization. J. Am. Chem. Soc. 2020, 142, 4254–4264.
Yang, Y.; Qin, H. Y.; Jiang, M. W.; Lin, L.; Fu, T.; Dai, X. L.; Zhang, Z. X.; Niu, Y.; Cao, H. J.; Jin, Y. Z. et al. Entropic ligands for nanocrystals: From unexpected solution properties to outstanding processability. Nano Lett. 2016, 16, 2133–2138.
Oh, W. D.; Dong, Z. L.; Lim, T. T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal.BEnviron. 2016, 194, 169–201.
Lee, J.; Von Gunten, U.; Kim, J. H. Persulfate-based advanced oxidation: Critical assessment of opportunities and roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081.
Liu, S.; Hassan, S. U.; Ding, H. J.; Li, S. Y.; Jin, F.; Miao, Z. Q.; Wang, X. X.; Li, H.; Zhao, C. Removal of sulfamethoxazole in water by electro-enhanced Co2+/peroxydisulfate system with activated carbon fiber-cathode. Chemosphere 2020, 245, 125644.
Shao, H. X.; Chen, J.; Xu, J. H.; Liu, Y.; Dong, H. Y.; Qiao, J. L.; Guan, X. H. Naproxen as a turn-on chemiluminescent probe for realtime quantification of sulfate radicals. Environ. Sci. Technol. 2023, 57, 8818–8827.
Ji, X. H.; Copenhaver, D.; Sichmeller, C.; Peng, X. G. Ligand bonding and dynamics on colloidal nanocrystals at room temperature: The case of alkylamines on CdSe nanocrystals. J. Am. Chem. Soc. 2008, 130, 5726–5735.
Hens, Z.; Martins, J. C. A solution NMR toolbox for characterizing the surface chemistry of colloidal nanocrystals. Chem. Mater. 2013, 25, 1211–1221.
Hartley, C. L.; Kessler, M. L.; Lassalle, C. Y. D.; Camp, A. M.; Dempsey, J. L. Effects of ligand shell composition on surface reduction in PbS quantum dots. Chem. Mater. 2021, 33, 8612–8622.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 22125405 and 22074131). The authors also would like to thank Mrs. Fang Chen from the Chemistry Instrumentation Centre of Chemistry Department at Zhejiang University for technical assistance with SEM and TEM measurements.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Sun, H., Cao, Z., Qin, H. et al. Ligand-controlled electrochemiluminescence generation from CdSe/CdS/ZnS core/shell/shell quantum dots. Nano Res. 17, 7776–7785 (2024). https://doi.org/10.1007/s12274-024-6707-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-024-6707-1