Abstract
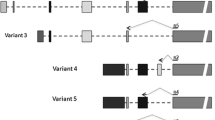
Numerous investigations have been recently published on the dysregulated expression of long-noncoding RNAs (lncRNAs) in various cancer types, emphasizing that abnormal lncRNA expression is a major contributor to tumourigenesis. A broad spectrum of lncRNAs is expressed in the central nervous system, where these RNAs seem to play key roles in brain development and function. In addition to expressing SOX2, a master regulator of pluripotency that lies within its third intron, lncRNA SOX2OT has a proposed role in regulating neural development. Based on our previous studies, alternative splicing of SOX2OT generates two alternatively spliced variants (SOX2OT-S1 and SOX2OT-S2). The present study investigated the expression patterns of SOX2OT variants and SOX2 in three principal types of brain tumours (gliomas, meningiomas and pituitary adenomas) and in four brain tumour cell lines (U87-MG, 1321N1, A172 and DAOY). Total RNA was extracted from 34 human brain tumour specimens, and the expression profile of target genes was measured using a real-time reverse transcription PCR approach. Our data revealed distinct expression patterns for SOX2OT variants and SOX2 in the brain tumour samples, indicating their potential involvement in brain tumourigenesis. Moreover, our results highlighted the potential usefulness of SOX2OT-S1, SOX2OT-S2, and SOX2 in molecular diagnosis and brain tumour classification.




Similar content being viewed by others
References
Amaral P. P., Christine N., Simon J. W., Marjan E. A., Susan M. S., Andrew C. P. et al. 2009 Complex architecture and regulated expression of the Sox2ot locus during vertebrate development. RNA 15, 2013–2027.
Annovazzi L., Mellai M., Caldera V., Valente G. and Schiffer D. 2011 SOX2 expression and amplification in gliomas and glioma cell lines. Cancer Genom. Proteom. 8, 139–147.
Atlasi Y., Mowla S. J., Ziaee S. A. M., Gokhale P. J. and Andrews W. P. 2008 OCT4 spliced variants are differentially expressed in human pluripotent and nonpluripotent cells. Stem Cells 26, 3068–3074.
Azad F. M., Taheri Bajgan E., Naeli P., Rudov A., Bagheri Moghadam M., Sadat Akhtar M. et al. 2022 Differential expression pattern of linc-ROR spliced variants in pluripotent and non-pluripotent cell lines. Cell J. 24, 569–576.
Banan R. and Christian H. 2017 The new WHO 2016 classification of brain tumors—what neurosurgeons need to know. Acta Neurochir. (Wien) 159, 403–418.
Bani-Yaghoub M., Roger G. T., Joy X. L., Dongling Z., Bogdan Z., Jagdeep K. S. et al. 2006 Role of Sox2 in the development of the mouse neocortex. Dev. Biol. 295, 52–66.
Bernard D., Kannanganattu V. P., Vidisha T., Sabrina C., Tetsuya N., Zhenyu X. et al. 2010 A long nuclear-retained non-coding RNA regulates synaptogenesis by modulating gene expression. EMBO J. 29, 3082–3093.
Bond A. M., Michael J. W. V., Evgeny A. S., Mary F. C., Julie C. S., John F. D. et al. 2009 Balanced gene regulation by an embryonic brain ncRNA is critical for adult hippocampal GABA circuitry. Nat. Neurosci. 12, 1020–1027.
Bush S. J., Lu C., Jaime M.T.-C. and Araxi O. U. 2017 Alternative splicing and the evolution of phenotypic novelty. Philos. Trans. R. Soc. B: Biol. Sci. 372, 1–7.
Bylund M., Elisabeth A., Bennett G. N. and Jonas M. 2003 Vertebrate neurogenesis is counteracted by Sox1–3 activity. Nat. Neurosci. 6, 1162–1168.
Chodroff R. A., Leo G., Tamara M. S., Peter L. O., Kay E. D., Eric D. G. et al. 2010 Long noncoding RNA genes: conservation of sequence and brain expression among diverse amniotes. Genome Biol. 11, 1–16.
Di Cristo G., Tommaso P., Laura C. and Evelyne S. 2011 GABAergic circuit development and its implication for CNS disorders. Neural. Plast. 2011, 1–2.
Dorton A. 2000 The pituitary gland: embryology, physiology, and pathophysiology. Neonatal. Netw. 19, 9–17.
Eaves C. J. 2008 Here, there, everywhere? Nature 456, 581–582.
Faghihi M. A., Farzaneh M., Ahmad M. K., Douglas E. W., Barbara G. S., Todd E. M. et al. 2008 Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of β-secretase. Nat. Med. 14, 723–730.
Fantes J., Nicola K. R., Sally-Ann L., Niolette I. M., Richard J. O. C., Patricia N. H. P. et al. 2003 Mutations in SOX2 cause anophthalmia. Nat. Genet. 33, 462–463.
Ferri A. L. M., Maurizio C., Daniela B., Antonello D. C., Annalisa C., Annamaria V. et al. 2004 Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Dev. 131, 3805–3819.
Finocchiaro G., Maria S. C., Stephanie F., Paola P., Valentina D. and Heiko M. 2007 Localizing hotspots of antisense transcription. Nucleic Acids Res. 35, 1488–1500.
Gangemi R. M. R., Fabrizio G., Daniela M., Marzia P., Maria C. C., Paolo M. et al. 2009 SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells 27, 40–48.
Gao N., Yueheng L., Jing L. I., Zhengfan G., Zhenzhen Y., Yong L. et al. 2020 Long non-coding RNAs: the regulatory mechanisms, research strategies, and future directions in cancers. Front. Oncol. 10, 1–13.
Gholipour A., Malakootian M. and Oveisee M. 2022 hsa-miR-508-5p as a New Potential Player in Intervertebral Disc Degeneration. Int. J. Mol. Cell Med. 11, 1–13.
Graham V., Jane K. H., Pamela E. and Larysa P. 2003 SOX2 functions to maintain neural progenitor identity. Neuron 39, 749–765.
Guo L., Meixiang S., Qingrui L., Xiaojie F., Xiao Zh. and Baoen S. 2013 The expression and clinical significance of melanoma-associated antigen-A1,-A3 and-A11 in glioma. Oncol. Lett. 6, 55–62.
Gutschner T. and Sven D. 2012 The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 9, 703–719.
Hartwell L., Mankoff D., Paulovich A., Ramsey S. and Swisher E. 2006 Cancer biomarkers: a systems approach. Nat. Biotechnol. 24, 905–908.
Hemmati H. D., Ichiro N., Jorge A. L., Michael M. S., Daniel H. G., Marianne B. F. et al. 2003 Cancerous stem cells can arise from pediatric brain tumors. Proc. Natl. Acad. Sci. USA 100, 15178–15183.
Hui A. B., Kwok-Wai L., Xiao-Lu Y., Wai-Sang P. and Ho-Keung N. 2001 Detection of multiple gene amplifications in glioblastoma multiforme using array-based comparative genomic hybridization. Lab. Invest. 81, 717–723.
Ikeda H. and Takashi Y. 2003 Immunohistochemical study of anaplastic meningioma with special reference to the phenotypic change of intermediate filament protein. Ann. Diagn. Pathol. 7, 214–222.
Kapranov P., Jill C., Sujit D., David A. N., Radharani D., Aarron T. W. et al. 2007 RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science 316, 1484–1488.
Kiefer J. C. 2007 Back to basics: Sox genes. Dev. Dyn. 236, 2356–2366.
Kim J., Jianlin C., Xiaohua S., Jianlong W. and Stuart H. O. 2008 An extended transcriptional network for pluripotency of embryonic stem cells. Cell 132, 1049–1061.
Kim S. H., Key-Hwan L., Sumin Y. and Jae-Yeol J. 2021 Long non-coding RNAs in brain tumors: roles and potential as therapeutic targets. J. Hematol. Oncol. 14, 1–17.
Korneev S. A., Elena I. K., Marya A. L., Sergei L. K., Giles C. and Michael O. 2008 Novel noncoding antisense RNA transcribed from human anti-NOS2A locus is differentially regulated during neuronal differentiation of embryonic stem cells. RNA 14, 2030–2037.
Li P. Y., Wang P., Gao S. G. and Dong D. Y. 2020 Long noncoding RNA SOX2-OT: regulations, functions, and roles on mental illnesses, cancers, and diabetic complications. Biomed. Res. Int. 2020, 1–12.
Louis D. N., Hiroko O., Otmar D. W., Webster K. C., Peter C. B., Anne J. et al. 2007 The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 114, 97–109.
Malakootian M., Mowla S. J., Saberi H., Asadi M. H., Atlasi Y. and Malekzadeh Shafaroudi A. 2010 Differential expression of nucleostemin, a stem cell marker, and its variants in different types of brain tumors. Mol. Carcinog. 49, 818–825.
Malakootian M., Mirzadeh Azad F., Naeli P., Pakzad M., Fouani Y., Taheri Bajgan E. et al. 2017 Novel spliced variants of OCT4, OCT4C and OCT4C1, with distinct expression patterns and functions in pluripotent and tumor cell lines. Eur. J. Cell Biol. 96, 347–355.
Malakootian M., Mirzadeh Azad F., Fouani Y., Taheri Bajgan E., Saberi H. and Mowla S. J. 2018 Anti-differentiation non-coding RNA, ANCR, is differentially expressed in different types of brain tumors. J. Neuro-Oncol. 138, 261–270.
Malakootian M., Gholipour A., Malekzadeh Shafaroud A., Arabian M. and Oveisee M. 2022 Potential roles of circular RNAs and environmental and clinical factors in intervertebral disc degeneration. Environ. Health Eng. Manag. 9, 189–200.
McFaline-Figueroa J. R. and Eudocia Q. L. 2018 Brain tumors. Am. J. Med. 131, 874–882.
Mehler M. F. and John S. M. 2006 Non-coding RNAs in the nervous system. Physiol. J. 575, 333–541.
Mehler M. F. and John S. M. 2007 Noncoding RNAs and RNA editing in brain development, functional diversification, and neurological disease. Physiol. Rev. 87, 799–823.
Mercer T. R., Marcel E. D., Jean M., Kenneth S. K., Mark F. M. and John S. M. 2008 Noncoding RNAs in long-term memory formation. Neuroscientist 14, 434–445.
Meseure D., Sophie V., François L., Kinan D. A., Rana H., Walid C. et al. 2016 Prognostic value of a newly identified MALAT1 alternatively spliced transcript in breast cancer. Br. J. Cancer 114, 1395–1404.
Mirzadeh Azad F., Malakootian M. and Mowla S. J. 2019 lncRNA PSORS1C3 is regulated by glucocorticoids and fine-tunes OCT4 expression in non-pluripotent cells. Sci. Rep. 9, 1–9.
Miyagi S., Tetsuichiro S., Ken-ichi M., Norihisa M., Yukiko G., Atsushi I. et al. 2004 The Sox-2 regulatory regions display their activities in two distinct types of multipotent stem cells. Mol. Cell. Biol. 24, 4207–4220.
Mizuseki K., Masashi K., Masaru M., Shigetada N. and Yoshiki S. 1998 Xenopus Zic-related-1 and Sox-2, two factors induced by chordin, have distinct activities in the initiation of neural induction. Development 125, 579–587.
Mohapatra G., Andrew W. B., Dong H. K., Kathleen L., Dan H. M., Michael D. P. et al. 1998 Genetic analysis of glioblastoma multiforme provides evidence for subgroups within the grade. Genes Chromosom. Cancer 21, 195–206.
Qian Y., Lei S. and Zhong L. 2020 Long non-coding RNAs in cancer: implications for diagnosis, prognosis, and therapy. Front. Med. (Lausanne) 902, 1–8.
Qureshi I. A. and Mark F. M. 2012 Emerging roles of non-coding RNAs in brain evolution, development, plasticity and disease. Nat. Rev. Neurosci. 13, 528–541.
Qureshi I. A., John S. M. and Mark F. M. 2010 Long non-coding RNAs in nervous system function and disease. Brain Res. 1338, 20–35.
Rodriguez J. M., Fernando P., Tomás D. D., Jesus V. and Michael L. T. 2020 An analysis of tissue-specific alternative splicing at the protein level. PLoS Comput. Biol. 16, 1–24.
Rynkeviciene R., Julija S., Egle S., Vaidotas S., Jurgita U., Edita M. K. et al. 2018 Non-coding RNAs in glioma. Cancers 11, 17–52.
Shahryari A., Mahmoud R. R., Youssef F., Nasrin A. O., Nader M. S., Mohammad S. et al. 2014 Two novel splice variants of SOX2OT, SOX2OT-S1, and SOX2OT-S2 are coupregulated with SOX2 and OCT4 in esophageal squamous cell carcinoma. Stem Cells 32, 126–134.
Singh S. K., Ian D. C., Mizuhiko T., Victoria E. B., Cynthia H., Jeremy S. et al. 2003 Identification of a cancer stem cell in human brain tumors. Cancer Res. 63, 5821–5828.
Statello L., Chun-Jie G., Ling-Ling C. and Maite H. 2021 Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 22, 96–118.
Uchikawa M., Yoshiko I., Tatsuya T., Yusuke K. and Hisato K. 2003 Functional analysis of chicken Sox2 enhancers highlights an array of diverse regulatory elements that are conserved in mammals. Dev. Cell 4, 509–519.
Uchikawa M., Tatsuya T., Yusuke K. and Hisato K. 2004 Efficient identification of regulatory sequences in the chicken genome by a powerful combination of embryo electroporation and genome comparison. Mech. Dev. 121, 1145–1158.
Wegner M. 1999 From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res. 27, 1409–1420.
Wegner M. and Claus C. S. 2005 From stem cells to neurons and glia: a Soxist’s view of neural development. Trends Neurosci. 28, 583–588.
Wei C. W., Ting L., Shan-Shan Z. and An-Shi W. 2018 The role of long noncoding RNAs in central nervous system and neurodegenerative diseases. Front. Behav. Neurosci. 12, 1–11.
Yao R. W., Yang W. and Ling-Ling C. 2019 Cellular functions of long noncoding RNAs. Nat. Cell Biol. 21, 542–551.
Yin J., Yanan S., Yanna S., Yuan Z., Jiayue D., Xiajuan H. et al. 2020 Knockdown of long non-coding RNA SOX2OT downregulates SOX2 to improve hippocampal neurogenesis and cognitive function in a mouse model of sepsis-associated encephalopathy. J. Neuroinflammation 17, 1–14.
Zappone M. V., Rossella G., Raffaella C., Natalia M., Silvia D. B., Elisabetta M. et al. 2000 Sox2 regulatory sequences direct expression of a (beta)-geo transgene to telencephalic neural stem cells and precursors of the mouse embryo, revealing regionalization of gene expression in CNS stem cells. Development 127, 2367–2382.
Acknowledgements
We wish to thank Mrs Nabian and Ms Esmaeili at the Brain and Spinal Injuries Research Center (Imam Khomeini Medical Center, Tehran) for their assistance in providing frozen clinical samples Funding was provided by Tarbiat Modares University. We are also grateful to Mr Saied Rahmani for his assistance in bioinformatics analysis and Ms Alipour for her technical assistance.
Author information
Authors and Affiliations
Contributions
Study conception or design: YF, SJM, and MM; bioinformatics analysis: AGH; data analysis and draft manuscript preparation: YF, MO, ASH, and HS; critical revision of the paper: MO, SJM, and MM; supervision of the research: SJM and MM; final approval of the version: YF, MO, AGH, ASH, HS, SJM, and MM.
Corresponding author
Additional information
Corresponding editor: Murali Dharan Bashyam
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fouani, Y., Gholipour, A., Oveisee, M. et al. Distinct gene expression patterns of SOX2 and SOX2OT variants in different types of brain tumours. J Genet 102, 25 (2023). https://doi.org/10.1007/s12041-023-01423-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12041-023-01423-z