Abstract
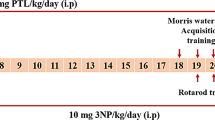
Laquinimod, an immunomodulatory agent under clinical development for Huntington disease (HD), has recently been shown to confer behavioural improvements that are coupled with prevention of atrophy of the white matter (WM)-rich corpus callosum (CC) in the YAC128 HD mice. However, the nature of the WM improvements is not known yet. Here we investigated the effects of laquinimod on HD-related myelination deficits at the cellular, molecular and ultrastructural levels. We showed that laquinimod treatment improves motor learning and motor function deficits in YAC128 HD mice, and confirmed its antidepressant effect even at the lowest dose used. In addition, we demonstrated for the first time the beneficial effects of laquinimod on myelination in the posterior region of the CC where it reversed changes in myelin sheath thickness and rescued Mbp mRNA and protein deficits. Furthermore, the effect of laquinimod on myelin-related gene expression was not region-specific since the levels of the Mbp and Plp1 transcripts were also increased in the striatum. Also, we did not detect changes in immune cell densities or levels of inflammatory genes in 3-month-old YAC128 HD mice, and these were not altered with laquinimod treatment. Thus, the beneficial effects of laquinimod on HD-related myelination abnormalities in YAC128 HD mice do not appear to be dependent on its immunomodulatory activity. Altogether, our findings describe the beneficial effects of laquinimod treatment on HD-related myelination abnormalities and highlight its therapeutic potential for the treatment of WM pathology in HD patients.






Similar content being viewed by others
References
Bates GP, Dorsey R, Gusella JF, Hayden MR, Kay C, Leavitt BR, Nance M, Ross CA, et al. (2015) Huntington disease. Nat Rev Dis Primers 15005. doi: https://doi.org/10.1038/nrdp.2015.5
Tai YF, Pavese N, Gerhard A, Tabrizi SJ, Barker RA, Brooks DJ, Piccini P (2007) Microglial activation in presymptomatic Huntington’s disease gene carriers. Brain 130:1759–1766. https://doi.org/10.1093/brain/awm044
Andre R, Carty L, Tabrizi SJ (2015) Disruption of immune cell function by mutant huntingtin in Huntington’s disease pathogenesis. Curr Opin Pharmacol 26:33–38. https://doi.org/10.1016/j.coph.2015.09.008
Crotti A, Glass CK (2015) The choreography of neuroinflammation in Huntington’s disease. Trends Immunol 36:364–373. https://doi.org/10.1016/j.it.2015.04.007
Denis HL, Lauruol F, Cicchetti F (2018) Are immunotherapies for Huntington’s disease a realistic option? Mol Psychiatry 16:889. https://doi.org/10.1038/s41380-018-0021-9
Sapp E, Kegel KB, Aronin N, Hashikawa T, Uchiyama Y, Tohyama K, Bhide PG, Vonsattel JP et al (2001) Early and progressive accumulation of reactive microglia in the Huntington disease brain. J Neuropathol Exp Neurol 60:161–172
Pavese N, Gerhard A, Tai YF, Ho AK, Turkheimer F, Barker RA, Brooks DJ, Piccini P (2006) Microglial activation correlates with severity in Huntington disease: a clinical and PET study. Neurology 66:1638–1643. https://doi.org/10.1212/01.wnl.0000222734.56412.17
Björkqvist M, Wild EJ, Thiele J, Silvestroni A, Andre R, Lahiri N, Raibon E, Lee RV et al (2008) A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington’s disease. J Exp Med 205:1869–1877. https://doi.org/10.1084/jem.20080178
Politis M, Pavese N, Tai YF, Kiferle L, Mason SL, Brooks DJ, Tabrizi SJ, Barker RA et al (2011) Microglial activation in regions related to cognitive function predicts disease onset in Huntington’s disease: a multimodal imaging study. Hum Brain Mapp 32:258–270. https://doi.org/10.1002/hbm.21008
Politis M, Lahiri N, Niccolini F, Su P, Wu K, Giannetti P, Scahill RI, Turkheimer FE et al (2015) Increased central microglial activation associated with peripheral cytokine levels in premanifest Huntington’s disease gene carriers. Neurobiol Dis 83:115–121. https://doi.org/10.1016/j.nbd.2015.08.011
Wild E, Magnusson A, Lahiri N, Krus U, Orth M, Tabrizi SJ, Björkqvist M (2011) Abnormal peripheral chemokine profile in Huntington’s disease. PLoS Curr 3:RRN1231. https://doi.org/10.1371/currents.RRN1231
Weiss A, Träger U, Wild EJ, Grueninger S, Farmer R, Landles C, Scahill RI, Lahiri N et al (2012) Mutant huntingtin fragmentation in immune cells tracks Huntington’s disease progression. J Clin Invest 122:3731–3736. https://doi.org/10.1172/JCI64565
Varrin-Doyer M, Zamvil SS, Schulze-Topphoff U (2014) Laquinimod, an up-and-coming immunomodulatory agent for treatment of multiple sclerosis. Exp Neurol 262PA:66–71. https://doi.org/10.1016/j.expneurol.2014.04.002
Kaye J, Piryatinsky V, Birnberg T, Hingaly T, Raymond E, Kashi R, Amit-Romach E, Caballero IS et al (2016) Laquinimod arrests experimental autoimmune encephalomyelitis by activating the aryl hydrocarbon receptor. PNAS 113:E6145–E6152. https://doi.org/10.1073/pnas.1607843113
Aharoni R, Saada R, Eilam R, Hayardeny L, Sela M, Arnon R (2012) Oral treatment with laquinimod augments regulatory T-cells and brain-derived neurotrophic factor expression and reduces injury in the CNS of mice with experimental autoimmune encephalomyelitis. J Neuroimmunol 251:14–24. https://doi.org/10.1016/j.jneuroim.2012.06.005
Thöne J, Ellrichmann G, Seubert S, Peruga I, Lee DH, Conrad R, Hayardeny L, Comi G et al (2012) Modulation of autoimmune demyelination by laquinimod via induction of brain-derived neurotrophic factor. Am J Pathol 180:267–274. https://doi.org/10.1016/j.ajpath.2011.09.037
Garcia-Miralles M, Hong X, Tan LJ, Caron NS, Huang Y, To XV, Lin RY, Franciosi S et al (2016) Laquinimod rescues striatal, cortical and white matter pathology and results in modest behavioural improvements in the YAC128 model of Huntington disease. Sci Rep 6:31652. https://doi.org/10.1038/srep31652
Garcia-Miralles M, Geva M, Tan JY, Yusof NABM, Cha Y, Kusko R, Tan LJ, Xu X et al (2017) Early pridopidine treatment improves behavioral and transcriptional deficits in YAC128 Huntington disease mice. JCI Insight 2. https://doi.org/10.1172/jci.insight.95665
Brooks SP, Dunnett SB (2009) Tests to assess motor phenotype in mice: a user’s guide. Nat Rev Neurosci 10:519–529. https://doi.org/10.1038/nrn2652
Pouladi MA, Graham RK, Karasinska JM, Xie Y, Santos RD, Petersen A, Hayden MR (2009) Prevention of depressive behaviour in the YAC128 mouse model of Huntington disease by mutation at residue 586 of huntingtin. Brain 132:919–932. https://doi.org/10.1093/brain/awp006
Paxinos G, Franklin KB (2012) Paxinos and franklin's the mouse brain in stereotaxic coordinates. Academic Press
Barazany D, Basser PJ, Assaf Y (2009) In vivo measurement of axon diameter distribution in the corpus callosum of rat brain. Brain 132(5):1210–1220
Pouladi MA, Stanek LM, Xie Y, Franciosi S, Southwell AL, Deng Y, Butland S, Zhang W et al (2012) Marked differences in neurochemistry and aggregates despite similar behavioural and neuropathological features of Huntington disease in the full-length BACHD and YAC128 mice. Hum Mol Genet 21:2219–2232. https://doi.org/10.1093/hmg/dds037
Van Raamsdonk JM, Murphy Z, Slow EJ, Leavitt BR, Hayden MR (2005) Selective degeneration and nuclear localization of mutant huntingtin in the YAC128 mouse model of Huntington disease. Hum Mol Genet 14:3823–3835. https://doi.org/10.1093/hmg/ddi407
Van Raamsdonk JM, Pearson J, Slow EJ, Hossain SM, Leavitt BR, Hayden MR et al (2005) Cognitive dysfunction precedes neuropathology and motor abnormalities in the YAC128 mouse model of Huntington’s disease. J Neurosci 25:4169–4180. https://doi.org/10.1523/JNEUROSCI.0590-05.2005
Carroll JB, Lerch JP, Franciosi S, Spreeuw A, Bissada N, Henkelman RM, Hayden MR (2011) Natural history of disease in the YAC128 mouse reveals a discrete signature of pathology in Huntington disease. Neurobiol Dis 43:257–265. https://doi.org/10.1016/j.nbd.2011.03.018
Teo RTY, Hong X, Yu-Taeger L, Huang Y, Tan LJ, Xie Y, To XV, Guo L et al (2016) Structural and molecular myelination deficits occur prior to neuronal loss in the YAC128 and BACHD models of Huntington disease. Hum Mol Genet 25:2621–2632. https://doi.org/10.1093/hmg/ddw122
Brück W, Pförtner R, Pham T, Zhang J, Hayardeny L, Piryatinsky V et al. (2012) Reduced astrocytic NF-κB activation by laquinimod protects from cuprizone-induced demyelination. Acta Neuropathologica 124(3):411–424. https://doi.org/10.1007/s00401-012-1009-1
Zwilling D, Huang S-Y, Sathyasaikumar KV, Notarangelo FM, Guidetti P, Wu HQ, Lee J, Truong J et al (2011) Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell 145:863–874. https://doi.org/10.1016/j.cell.2011.05.020
Ehrnhoefer DE, Caron NS, Deng Y, Qiu X, Tsang M, Hayden MR (2016) Laquinimod decreases Bax expression and reduces caspase-6 activation in neurons. Exp Neurol 283:121–128. https://doi.org/10.1016/j.expneurol.2016.06.008
Xiang Z, Valenza M, Cui L, Leoni V, Jeong HK, Brilli E, Zhang J, Peng Q et al (2011) Peroxisome-proliferator-activated receptor gamma coactivator 1 α contributes to dysmyelination in experimental models of Huntington’s disease. J Neurosci 31:9544–9553. https://doi.org/10.1523/JNEUROSCI.1291-11.2011
Jin J, Peng Q, Hou Z, Jiang M, Wang X, Langseth AJ, Tao M, Barker PB et al (2015) Early white matter abnormalities, progressive brain pathology and motor deficits in a novel knock-in mouse model of Huntington’s disease. Hum Mol Genet 24:2508–2527. https://doi.org/10.1093/hmg/ddv016
Baumann N, Pham-Dinh D (2001) Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 81:871–927
Jahn O, Tenzer S, Werner HB (2009) Myelin proteomics: molecular anatomy of an insulating sheath. Mol Neurobiol 40:55–72. https://doi.org/10.1007/s12035-009-8071-2
Shackleford G, Sampathkumar NK, Hichor M et al (2018) Involvement of Aryl hydrocarbon receptor in myelination and in human nerve sheath tumorigenesis. PNAS 115:E1319–E1328. https://doi.org/10.1073/pnas.1715999115
Acknowledgements
Microscopy images for this study were acquired at the SBIC-Nikon Imaging Centre (Biopolis, Singapore).
Funding
This study was supported by a grant from Teva Pharmaceuticals. M.A.P. is supported by a Strategic Positioning Fund for Genetic Orphan Diseases (SPF2012/005) from the Agency for Science Technology and Research, and by the National University of Singapore, Singapore.
Author information
Authors and Affiliations
Contributions
M.G.M. designed and performed experiments, data analysis and interpretation, and wrote the manuscript. N.A.B.M.Y., J.Y.T., C.R., H.S. and L.J.T. performed experiments. N.Z. and M.R.H. contributed to the study design and revision of the manuscript. M.A.P. conceived and designed experiments, participated in analysis and interpretation of data, and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
N.Z., H.B. and M.R.H. are employees of Teva Pharmaceuticals, and contributed to the study design and revision of the manuscript. Teva Pharmaceuticals played no role in the treatment or testing of animals, or the collection, analysis and interpretation of the results.
Electronic Supplementary Material
ESM 1
(PDF 89 kb)
Rights and permissions
About this article
Cite this article
Garcia-Miralles, M., Yusof, N.A.B.M., Tan, J.Y. et al. Laquinimod Treatment Improves Myelination Deficits at the Transcriptional and Ultrastructural Levels in the YAC128 Mouse Model of Huntington Disease. Mol Neurobiol 56, 4464–4478 (2019). https://doi.org/10.1007/s12035-018-1393-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1393-1