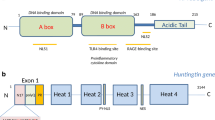
Abstract
Huntington’s disease (HD) is a neurodegenerative syndrome caused by mutations of the IT15 gene encoding for the huntingtin protein. Some research groups have previously shown that HD is associated with cellular radiosensitivity in quiescent cells. However, there is still no mechanistic model explaining such specific clinical feature. Here, we examined the ATM-dependent signaling and repair pathways of the DNA double-strand breaks (DSB), the key damage induced by ionizing radiation, in human HD skin fibroblasts. Early after irradiation, quiescent HD fibroblasts showed an abnormally low rate of recognized DSB managed by non-homologous end-joining reflected by a low yield of nuclear foci formed by phosphorylated H2AX histones and by 53BP1 protein. Furthermore, HD cells elicited a significant but moderate yield of unrepaired DSB 24 h after irradiation. Irradiated HD cells also presented a delayed nucleo-shuttling of phosphorylated forms of the ATM kinase, potentially due to a specific binding of ATM to mutated huntingtin in the cytoplasm. Our results suggest that HD belongs to the group of syndromes associated with a low but significant defect of DSB signaling and repair defect associated with radiosensitivity. A combination of biphosphonates and statins complements these impairments by facilitating the nucleo-shuttling of ATM, increasing the yield of recognized and repaired DSB.








Similar content being viewed by others
Notes
The name chorea was historically given by Paracelsius to any disease associated with disordered movements.
References
Huntington G (1872) On chorea. Med Surg Report 26(15):1
Zheng Z, Diamond MI (2013) Huntington disease and the huntingtin protein. Prog Mol Biol Transl Sci 107:189–214
Pringsheim T, Wiltshire K, Day L, Dykeman J, Steeves T, Jette N (2012) The incidence and prevalence of Huntington’s disease: a systematic review and meta-analysis. Mov Disord 27(9):1083–1091
van der Burg JM, Bjorkqvist M, Brundin P (2009) Beyond the brain: widespread pathology in Huntington’s disease. Lancet Neurol 8(8):765–774
Rubinsztein DC, Barton DE, Davison BC, Ferguson-Smith MA (1993) Analysis of the huntingtin gene reveals a trinucleotide-length polymorphism in the region of the gene that contains two CCG-rich stretches and a correlation between decreased age of onset of Huntington’s disease and CAG repeat number. Hum Mol Genet 2(10):1713–1715
Rubinsztein DC, Leggo J, Barton DE, Ferguson-Smith MA (1993) Site of (CCG) polymorphism in the HD gene. Nat Genet 5(3):214–215
Cisbani G, Cicchetti F (2012) An in vitro perspective on the molecular mechanisms underlying mutant huntingtin protein toxicity. Cell Death Dis 3:e382
Jonson I, Ougland R, Larsen E (2013) DNA repair mechanisms in Huntington’s disease. Mol Neurobiol 47:1093–1102
Enokido Y, Tamura T, Ito H, Arumughan A, Komuro A, Shiwaku H, Sone M, Foulle R, Sawada H, Ishiguro H, Ono T, Murata M, Kanazawa I, Tomilin N, Tagawa K, Wanker EE, Okazawa H (2010) Mutant huntingtin impairs Ku70-mediated DNA repair. J Cell Biol 189:425–443
Arlett CF (1980) Presymptomatic diagnosis of Huntington’s disease? Lancet 1(8167):540
Arlett CF, Priestley A (1984) Deficient recovery from potentially lethal damage in some gamma-irradiated human fibroblast cell strains. Br J Cancer Suppl 6:227–232
Arlett CF, Muriel WJ (1979) Radiosensitivity in Huntington’s disease. Heredity 42:276
Moshell AN, Tarone RE, Barrett SF, Robbins JH (1980) Radiosensitivity in Huntington’s disease: implications for pathogenesis and presymptomatic diagnosis. Lancet 1(8158):9–11
McGovern D, Webb T (1982) Sensitivity to ionising radiation of lymphocytes from Huntington’s chorea patients compared to controls. J Med Genet 19(3):168–174
Deschavanne PJ, Fertil B (1996) A review of human cell radiosensitivity in vitro. Int J Radiat Oncol Biol Phys 34(1):251–266
Jeggo PA, Lobrich M (2007) DNA double-strand breaks: their cellular and clinical impact? Oncogene 26(56):7717–7719
Joubert A, Gamo K, Bencokova Z, Gastaldo J, Rénier W, Chavaudra N, Favaudon V, Arlett C, Foray N (2008) DNA double-strand break repair defects in syndromes associated with acute radiation response: at least two different assays to predict intrinsic radiosensitivity? Int J Radiat Biol 84(2):1–19
Rothkamm K, Lobrich M (2003) Evidence for a lack of DNA double-strand break repair in human cells exposed to very low X-ray doses. Proc Natl Acad Sci U S A 100(9):5057–5062
Bodgi L, Granzotto A, Devic C, Vogin G, Lesne A, Bottollier-Depois JF, Victor JM, Maalouf M, Fares G, Foray N (2013) A single formula to describe radiation-induced protein relocalization: towards a mathematical definition of individual radiosensitivity. J Theor Biol 333:135–145
Foray N, Priestley A, Alsbeih G, Badie C, Capulas EP, Arlett CF, Malaise EP (1997) Hypersensitivity of ataxia telangiectasia fibroblasts to ionizing radiation is associated with a repair deficiency of DNA double-strand breaks. Int J Radiat Biol 72(3):271–283
Foray N, Marot D, Gabriel A, Randrianarison V, Carr AM, Perricaudet M, Ashworth A, Jeggo P (2003) A subset of ATM- and ATR-dependent phosphorylation events requires the BRCA1 protein. EMBO J 22(11):2860–2871
Varela I, Pereira S, Ugalde AP, Navarro CL, Suarez MF, Cau P, Cadinanos J, Osorio FG, Foray N, Cobo J, de Carlos F, Levy N, Freije JM, Lopez-Otin C (2008) Combined treatment with statins and aminobisphosphonates extends longevity in a mouse model of human premature aging. Nat Med 14(7):767–772
Renier W, Joubert A, Bencokova Z, Gastaldo J, Massart C, Foray N (2007) Consequences of the bleed-through phenomenon in immunofluorescence of proteins forming radiation-induced nuclear foci. Int J Radiat Biol 83(8):543–549
Cattaneo E, Zuccato C, Tartari M (2005) Normal huntingtin function: an alternative approach to Huntington’s disease. Nat Rev Neurosci 6(12):919–930
Maison C, Almouzni G (2004) HP1 and the dynamics of heterochromatin maintenance. Nat Rev Mol Cell Biol 5(4):296–304
Dinant C, Luijsterburg MS (2009) The emerging role of HP1 in the DNA damage response. Mol Cell Biol 29(24):6335–6340
Nielsen AL, Oulad-Abdelghani M, Ortiz JA, Remboutsika E, Chambon P, Losson R (2001) Heterochromatin formation in mammalian cells: interaction between histones and HP1 proteins. Mol Cell 7(4):729–739
Hegde ML, Mantha AK, Hazra TK, Bhakat KK, Mitra S, Szczesny B (2012) Oxidative genome damage and its repair: implications in aging and neurodegenerative diseases. Mech Ageing Dev 133(4):157–168
Grote SJ, Joshi GP, Revell SH, Shaw CA (1981) Observations of radiation-induced chromosome fragment loss in live mammalian cells in culture, and its effect on colony-forming ability. Int J Radiat Biol Relat Stud Phys Chem Med 39(4):395–408
Fertil B, Malaise EP (1981) Inherent cellular radiosensitivity as a basic concept for human tumor radiotherapy. Int J Radiat Oncol Biol Phys 7(5):621–629
Mochan TA, Venere M, DiTullio RA Jr, Halazonetis TD (2004) 53BP1, an activator of ATM in response to DNA damage. DNA Repair (Amst) 3(8–9):945–952
Bakkenist CJ, Kastan MB (2003) DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421(6922):499–506
Cornforth MN, Bedford JS (1987) A quantitative comparison of potentially lethal damage repair and the rejoining of interphase chromosome breaks in low passage normal human fibroblasts. Radiat Res 111(3):385–405
Illuzzi J, Yerkes S, Parekh-Olmedo H, Kmiec EB (2009) DNA breakage and induction of DNA damage response proteins precede the appearance of visible mutant huntingtin aggregates. J Neurosci Res 87(3):733–747
Jeon GS, Kim KY, Hwang YJ, Jung M-K, An S, Ouchi M, Ouchi T, Kowall N, Lee J, Ryu H (2012) Deregulation of BRCA1 leads to impaired spatiotemporal dynamics of γ-H2AX and DNA damage responses in Huntington’s disease. Mol Neurobiol 45:550–563
Lambert WC, Gagna CE, Lambert MW (2008) Xeroderma pigmentosum: its overlap with trichothiodystrophy, Cockayne syndrome and other progeroid syndromes. Adv Exp Med Biol 637:128–137
Barlow C, Ribaut-Barassin C, Zwingman TA, Pope AJ, Brown KD, Owens JW, Larson D, Harrington EA, Haeberle AM, Mariani J, Eckhaus M, Herrup K, Bailly Y, Wynshaw-Boris A (2000) ATM is a cytoplasmic protein in mouse brain required to prevent lysosomal accumulation. Proc Natl Acad Sci U S A 97(2):871–876
Valenza M, Rigamonti D, Goffredo D, Zuccato C, Fenu S, Jamot L, Strand A, Tarditi A, Woodman B, Racchi M, Mariotti C, Di Donato S, Corsini A, Bates G, Pruss R, Olson JM, Sipione S, Tartari M, Cattaneo E (2005) Dysfunction of the cholesterol biosynthetic pathway in Huntington’s disease. J Neurosci 25(43):9932–9939
del Toro D, Xifro X, Pol A, Humbert S, Saudou F, Canals JM, Alberch J (2010) Altered cholesterol homeostasis contributes to enhanced excitotoxicity in Huntington’s disease. J Neurochem 115(1):153–167
Beverstock GC, Simons JW (1982) Study on the variation in X-ray sensitivity of human diploid fibroblast. Normal radiosensitivity of cells derived from patients with Huntington’s disease. Mutat Res 96:75–87
Nove J, Tarone RE, Little JB, Robbins JH (1987) Radiation sensitivity of fibroblast strains from patients with Usher’s syndrome, Duchenne muscular dystrophy, and Huntington’s disease. Mutat Res 184(1):29–38
Acknowledgments
We thank Madame Beaufrère for her assistance in editing the English. M.L.F. was supported by the Société Française de Radioprotection (SFRP). We thank the Association Pour la Recherche sur l'Ataxie-Telangiectasie (APRAT), the Electricité de France (Comité de Radioprotection), the Plan Cancer/AVIESAN “Micromegas project, the ANR “Hemi-breaks T” project, the Centre National d’Etudes Spatiales (CNES), and the Commissariat Général à l’Investissement (INDIRA project).
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is dedicated to the memory of Edmond-Philippe Malaise, pioneer of the notion of individual radiosensitivity.
Rights and permissions
About this article
Cite this article
Ferlazzo, M.L., Sonzogni, L., Granzotto, A. et al. Mutations of the Huntington’s Disease Protein Impact on the ATM-Dependent Signaling and Repair Pathways of the Radiation-Induced DNA Double-Strand Breaks: Corrective Effect of Statins and Bisphosphonates. Mol Neurobiol 49, 1200–1211 (2014). https://doi.org/10.1007/s12035-013-8591-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8591-7