Abstract
Insulin receptors in the brain are found in high densities in the hippocampus, a region that is fundamentally involved in the acquisition, consolidation, and recollection of new information. Using the intranasal method, which effectively bypasses the blood–brain barrier to deliver and target insulin directly from the nose to the brain, a series of experiments involving healthy humans has shown that increased central nervous system (CNS) insulin action enhances learning and memory processes associated with the hippocampus. Since Alzheimer's disease (AD) is linked to CNS insulin resistance, decreased expression of insulin and insulin receptor genes and attenuated permeation of blood-borne insulin across the blood–brain barrier, impaired brain insulin signaling could partially account for the cognitive deficits associated with this disease. Considering that insulin mitigates hippocampal synapse vulnerability to amyloid beta and inhibits the phosphorylation of tau, pharmacological strategies bolstering brain insulin signaling, such as intranasal insulin, could have significant therapeutic potential to deter AD pathogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Insulin's Scientific Journey from the Periphery to the Brain: a Short Historical Overview
In 1916, the Romanian scientist Nicolae Paulescu showed for the first time that insulin, when injected into diabetic dogs, had a normalizing effect on blood sugar levels [1]. Further research revealed that the ability of certain tissues, such as the skeletal muscle, to utilize glucose essentially depends on the insulin signal [2], while that of the brain was considered insulin-independent [3]. As a consequence, the general scientific interest in the role insulin might play for brain functions abated. This interest was only revived when insulin receptors (IRs) were ubiquitously detected in both rodent and human brains [4–6]. The fact that IRs are widely expressed in the brain, combined with evidence that insulin crosses the blood–brain barrier (BBB) by a saturable, receptor-mediated transport mechanism [7], challenged prevailing scientific concepts that the brain would not be sensitive to insulin, and thus reawakened the scientific interest related to the influence of this hormone on brain functions.
IRs are found in particularly high densities in the hippocampus [4]. This central site is fundamentally involved in the acquisition, consolidation, and recollection of information [8]. In the next section, we will summarize current evidence suggesting that brain insulin signaling influences learning and memory under physiological-like conditions. It is important to keep in mind that insulin also affects neural circuits involved in the regulation of food intake and energy expenditure (e.g., [9–11]). In order to gain a comprehensive overview regarding the impact of brain insulin pathways on whole-body energy homeostasis, the interested reader is referred to [12–14].
Effects of Insulin on Memory Processing: from the Needle to the Nose
Hippocampal damage causes severe impairments in the ability of rodents to learn and remember a location in space [15], highlighting that the hippocampus is an essential support for place memory. Using the intracerebroventricular route, several studies have consistently proven that insulin benefits spatial memory formation by acting at hippocampal sites [16, 17]. For instance, rats receiving an intracerebroventricular injection of insulin after they had been shocked when entering a darkened compartment show an increased latency to enter the same place in a recall session 24 h later [17]. This suggests that insulin enhances memory for the negative consequences associated with spatial orientation.
In humans, it has been shown that postprandial-like increases in plasma insulin levels are followed by increases in cerebrospinal fluid levels of this hormone, indicating that plasma insulin accesses the brain, and therefore potentially affects memory function in humans [18]. Consistent with this assumption, euglycemic hyperinsulinemic clamps have shown that higher, compared to lower doses of intravenous insulin improved verbal memory performance in healthy young men [19]. There are, however, some caveats with this method which limit conclusive interpretation of the data. The continuous glucose infusion during the hyperinsulinemic clamp procedure to ensure euglycemia may exert a biasing impact on cognitive functioning. Moreover, systemic insulin infusions acutely increase serum cortisol levels in healthy men [20], a hormone which is known to influence core mechanisms of memory processing [21].
One way to minimize these methodological biases and reduce or avoid systemic exposure to insulin while targeting the brain is intranasal administration. The efficacy of the intranasal administration route to deliver substances directly to the brain has been proven in various experimental settings. For instance, in 1970, in a landmark study, de Lorenzo demonstrated in squirrel monkeys that intranasally administered gold particles translocated from the nasal cavity to the olfactory bulb. In 1986, experiments in mouse, rat, and squirrel monkey expanded these results by showing that intranasally administered horseradish peroxidase passed freely through intercellular junctions of the olfactory epithelia to reach the olfactory bulbs of the CNS extracellularly within 45–90 min [22]. In 1989, Frey first proposed the noninvasive intranasal method for bypassing the BBB to target therapeutic proteins, growth factors, and hormones (including insulin) to the brain to treat neurodegenerative disorders such as Alzheimer's disease [23, 24] and later expanded on the specific use of intranasal insulin to target the brain to treat Alzheimer's disease and other CNS disorders [25, 26]. In 2004, Thorne et al. demonstrated in rodents that intranasal insulin-like growth factor I not only bypassed the BBB to reach the brain within 30 min but did so by traveling extracellularly along both the olfactory and trigeminal neural pathways [27]. The mechanisms involved in the direct intranasal delivery and targeting of therapeutics to the CNS that allow drugs to bypass the BBB have been reviewed elsewhere [28]. Using this method, Born et al. were the first to demonstrate that intranasally administered neuropeptides, including insulin and melanocortin [4–10], bypass the bloodstream to reach the cerebrospinal fluid within 10 min ([29]; Fig. 1). Experiments in humans have shown that intranasal insulin exerts rapid effects on EEG parameters [30, 31], indicating that following intranasal administration, a significant amount of insulin reaches the brain in a functionally active state. In the next section, we will highlight evidence demonstrating that intranasal insulin improves memory function in healthy young humans.
Sniffing insulin increases CSF insulin concentrations in humans [data from [29]]. Concentrations of insulin in cerebrospinal fluid (CSF) before and within 80 min after intranasal administration of human insulin (40 IU; solid lines, n = 8) and placebo (dashed lines, n = 5) Substances were administered with a nasal spray atomizer. Nose symbol indicates time of substance administration. Means ± SEM are indicated. **P value smaller than 0.01, *P value smaller than 0.05, for pairwise comparisons of baseline adjusted values between both conditions
Intranasal Insulin: a Memory Enhancer in Humans?
To examine whether enhanced brain insulin signaling affects hippocampus-dependent memory processing in humans, in a previous intranasal experiment, 38 students of normal body weight (body mass index (BMI) <25 kg/m²; 14 females) were assigned into two groups receiving intranasally either placebo or regular human insulin (160 IU/day) during an 8-week treatment phase [32]. At the beginning and end of treatment, i.e., after the first intranasal administration and after 7 weeks of intranasal treatment, lists of 30 words were verbally presented to the subjects and immediate recall was measured after a distraction interval of 3 min. In a delayed recall, which took place 1 week later, subjects wrote down all words they could remember from the list presented before. In comparison to placebo, delayed recall of words significantly improved after 8 weeks of intranasal insulin administration whereas immediate recall was generally not affected (Fig. 2a). This was the first demonstration that intranasal insulin improves memory in humans. Blood glucose and plasma insulin levels did not differ between the placebo and insulin groups. Employing the same study design as presented above [32], these results have been confirmed in additional experiments involving either obese men treated with regular human insulin [33], or normal weight men treated with the insulin analogue insulin aspart [34]. While the delayed onset of insulin's action on memory points to the involvement of more gradual plastic processes determining neuronal function, recent data also indicate that a single dose of intranasal insulin acutely improves hippocampus-dependent memory in both young [9] and middle-aged [35] women. These experimental data clearly support a role of brain insulin signaling pathways in the formation of hippocampus-dependent memory in healthy humans.
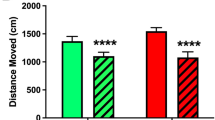
a Intranasal insulin improves memory in humans [data from [32]]. Immediate and delayed word list recall in healthy humans following 7 (immediate recall) and 8 weeks (delayed recall) of intranasal administration of regular human insulin (160 IU/day, black bars, n = 19) and placebo (white bars, n = 19), respectively. Immediate recall was tested 3 min after the auditory presentation of 30 nouns (e.g., car, tree, and chocolate). Delayed recall of the same list of nouns was tested after one more week of treatment. Baseline adjusted means ± SEM are indicated. b Intranasal insulin improves memory in patients with MCI or in the early stages of AD [data from [55]]. Mean memory saving scores (±SEM) in patients with early stage Alzheimer's disease and mild cognitive impairment (MCI) at baseline (day 0) and after 21 days of treatment with placebo (n = 12) and insulin (2 × 20 IU/day; n = 13), respectively. Insulin-treated patients showed increased memory savings over the 21-day period relative to placebo. *P value smaller than 0.05
Insulin Resistance, Cognitive Function, and Alzheimer's Disease: Is There a Link?
Insulin resistance, a key metabolic disturbance of type 2 diabetes, is characterized by reduced responsiveness of target tissues to the insulin signal. Results from population-based studies suggest that insulin resistance is tightly linked to cognitive impairment and smaller brain size [36, 37]. These epidemiological data have been supplemented by recent results showing that men with enhanced peripheral insulin resistance have reduced spontaneous cortical activity, as assessed by magnetoencephalography during euglycaemic insulin infusion [38]. In this study, intravenous infusions of insulin in insulin-resistant subjects induced no changes in beta band activity (commonly associated with increased cortical processing activity; [39]) and even a decrease of theta band activity (well known to be related to increased memory performance; [40]) as assessed by magnetoencephalography. In contrast, insulin-sensitive controls showed increased activity in both beta and theta bands in response to intravenous insulin [38]. Further, as revealed by fludeoxyglucose F 18 positron emission tomography (PET), greater insulin resistance has also been linked to reduced cerebral glucose metabolic rates in frontal, parietotemporal, and cingulate regions in elderly, a pattern associated with Alzheimer's disease [41]. These findings therefore provide important evidence supporting the hypothesis that brain insulin signaling is of biological significance for memory functions in humans. This might not be surprising as there is some evidence, albeit limited, that insulin facilitates regional glucose uptake and utilization in the brain [42].
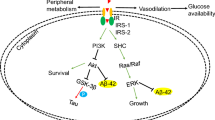
Remarkably, disturbances in brain insulin signaling have been previously linked to Alzheimer's diseases (AD) [43]. While one study of Japanese patients with AD reported increased fasting levels of cerebrospinal fluid (CSF) insulin [44], other studies have shown that patients suffering from AD show reduced brain IR activity along with lower CSF insulin levels and peripheral hyperinsulinemia [45, 46] and attenuated insulin and insulin-like growth factor receptor expression [47]. This suggests that disrupted brain insulin signaling may contribute to the loss of memory functions associated with this disease. Since insulin can mitigate the synaptic loss of hippocampal neurons induced by amyloid beta [48] and regulate glycogen synthase kinase-3 beta, thereby inhibiting the phosphorylation of tau protein that forms neurofibrillary tangles which are a further pathological feature of AD [49], impaired CNS signaling of the hormone may also promote brain atrophy associated with AD [50]. Against this background, strategies that increase brain insulin concentrations may be expected to counteract AD-related memory deficits and may have therapeutic potential to deter AD pathogenesis. Supporting this assumption, enhancing brain insulin levels in AD patients by intravenous insulin administration has been shown to acutely improve performance on a hippocampus-dependent memory task [51]. However, owing to the notion that high systemic doses would be needed to achieve functionally effective insulin concentrations in the brain, this mode of administration is not viable in the clinical setting. In contrast, intranasal insulin is a promising approach enabling the selective elevation of CNS insulin levels while circumventing risks associated with peripheral administration. As detailed in the next section, convincing evidence has been presented that supports a beneficial effect of intranasal insulin on hippocampus-dependent memory functions in patients with early AD or its prodrome, amnestic mild cognitive impairment (MCI).
Enhancing Brain Insulin Signaling Improves Memory Functions in AD Patients
In a study published in 2006, Reger and coworkers evaluated acute effects of a single intranasal administration of insulin (20 and 40 IU, respectively) on the ability of cognitively impaired patients (i.e., AD and MCI) to recall parts of a story [52]. In detail, subjects heard a brief narrative containing 44 parts of information from a story and were asked to recall as much as possible both immediately and after a 10-min delay. Total verbatim recall was scored. After intranasal administration of either dose of insulin, story recall was particularly improved in those patients who were Epsilon 4 (ε4) negative, with highest performance after 20 IU. The ε4 allele belongs to the apolipoprotein E gene and represents a strong genetic risk factor for late-onset AD [53]. These beneficial effects of intranasal insulin on verbal memory performance were confirmed in separate experiments in that insulin-treated patients retained more verbal information compared with the placebo-assigned group (see also Fig. 2b; [54, 55]). In a very recently published clinical trial including 104 adults with amnestic MCI or mild to moderate AD, 4 months of intranasal insulin administration (20 IU insulin/day) preserved not only general cognition but also improved the metabolic integrity of the brain, as indicated by a fludeoxyglucose F 18 PET [56]. These results suggest that targeting the brain insulin pathway by means of intranasal administration of the hormone is a promising therapeutic strategy to improve memory and potentially deter the process of this devastating disease. Of note, in two of these studies, benefits in episodic memory were found only in those who received a daily dose of 20 IU insulin, while those who received 40 IU did not show an improved recall of episodic memory [54, 56]. These data suggest that intranasal insulin may dose-dependently improve the recall of episodic memories in AD patients. However, it might be also that a larger sample size or a larger set of cognitive tests may also show beneficial effects of 40 IU insulin on episodic memory. Thus, more research to address these issues is needed.
At this point, it is important to emphasize that AD and MCI patients carrying at least one copy of the ε4 allele did not benefit from treatment [52, 54]. One possible explanation for these discrepant results could be that dose–response curves differ by apolipoprotein E genotype. This view is supported by previous findings showing that AD patients without ε4 showed both lower insulin-mediated glucose disposal rates and significant memory facilitation in the hyperinsulinemic condition than did AD patients carrying at least one copy of the ε4 allele [57], suggesting that defective insulin action may be of particular pathophysiologic significance for patients without an epsilon 4 allele. Thus, it is possible that even the lowest intranasal insulin dose was still too high to induce memory benefits in ε4+ subjects. Alternatively, treatment response differences between APOE groups may reflect differences in the amount of insulin transported to the CNS following intranasal administration. Future research is required to explore these possibilities.
Nevertheless, these findings have piloted an approach that may improve memory function in patients with AD and support further investigation of the benefits of intranasal insulin for patients with Alzheimer's disease. An alternative strategy, which may overcome central insulin resistance in AD that warrants further exploration, involves improving the brain's response to the insulin signal. As mentioned earlier, patients suffering from AD show not only lower CSF insulin levels [45] but also a reduced IR activity [46], which could represent a promising target for therapeutic intervention. Insulin sensitizers like the peroxisome proliferator activate receptor gamma agonist rosiglitazone, for example, which might exert its enhancing effects on insulin sensitivity not only in the periphery but also in the CNS. Accordingly, an improved memory function in patients with AD was observed under treatment with rosiglitazone [58]. However, these findings have not been confirmed in a subsequent clinical trial [59], highlighting the need to find alternative strategies to counteract the reduced brain IR activity in AD patients.
Clinical Safety of Intranasal Insulin Administration
Peripheral insulin elevations have been previously linked to synchronous increases in circulating and CSF concentrations of amyloid beta [60], elevated blood pressure [61], and enhanced hypothalamo–pituitary–adrenal [20] secretory activity. In contrast, subchronic elevations of CNS insulin concentrations by intranasal administration of the hormone have been associated with reduced circulating concentrations of amyloid beta [55], no changes in blood pressure [62], and dampened hypothalamo–pituitary–adrenal (HPA) secretory activity [32], indicating that shorter periods of intranasal insulin apparently do not produce harmful side effects in humans. However, as there is evidence linking reduced insulin signaling in the rodent brain to longevity [63], larger studies of longer duration are definitely needed to allow final conclusions regarding the clinical safety of insulin treatment in AD.
Concluding Remarks
Approximately six decades after insulin was discovered by Nicolae Paulesco [1], insulin receptors were ubiquitously found in the CNS [4] and thus challenged prevailing concepts that the brain is an insulin insensitive organ. In aggregate, the studies briefly summarized above clearly support the idea that brain insulin signaling contributes to hippocampus-dependent learning and memory processes in humans. Further, these studies suggest that enhancing insulin signaling in the brain by means of intranasal administration may be a useful therapeutic option to overcome the CNS insulin resistance found in AD. Employing the combination of insulin sensitizers with intranasal insulin might be another fruitful research direction in order to treat CNS insulin resistance found in AD.
However, some caution is needed when interpreting the effects of intranasal insulin on memory in AD patients. Although promising, the observed effects were small in size in absolute terms and the treatment period was relatively short compared to the duration of AD, and therefore needs confirmation in larger studies of longer duration, in order to fully evaluate the therapeutic potential of intranasal insulin in the treatment of AD. Further, it cannot be ruled out that CNS insulin resistance, under yet unknown pathological conditions, might represent a homeostatic mechanism to counteract chronically elevated CSF concentrations of insulin.
References
Paulesco NC (1921) Recherche sur le rôle du pancréas dans l'assimilation nutritive. Archives Internationales de Physiologie 17:85–103
Krahl ME (1951) The effect of insulin and pituitary hormones on glucose uptake in muscle. Ann N Y Acad Sci 54(4):649–670
Park CR, Johnson LH (1955) Effect of insulin on transport of glucose and galactose into cells of rat muscle and brain. Am J Physiol 182(1):17–23
Havrankova J, Roth J, Brownstein M (1978) Insulin receptors are widely distributed in the central nervous system of the rat. Nature 272:827–829
Potau N, Escofet MA, Martinez MC (1991) Ontogenesis of insulin receptors in human cerebral cortex. J Endocrinol Invest 14:53–58
Sara VR, Hall K, Von HH, Humbel R, Sjogren B, Wetterberg L (1982) Evidence for the presence of specific receptors for insulin-like growth factors 1 (IGE-1) and 2 (IGF-2) and insulin throughout the adult human brain. Neurosci Lett 34:39–44
Woods SC, Seeley RJ, Baskin DG, Schwartz MW (2003) Insulin and the blood–brain barrier. Curr Pharm Des 9:795–800
Eichenbaum H (2004) Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron 44:109–120
Benedict C, Kern W, Schultes B, Born J, Hallschmid M (2008) Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab 93(4):1339–1344
Benedict C, Brede S, Schiöth HB, Lehnert H, Schultes B, Born J, Hallschmid M (2011) Intranasal insulin enhances postprandial thermogenesis and lowers postprandial serum insulin levels in healthy men. Diabetes 60(1):114–118
Hallschmid M, Benedict C, Schultes B, Fehm HL, Born J, Kern W (2004) Intranasal insulin reduces body fat in men but not in women. Diabetes 53(11):3024–3029
Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG (2000) Central nervous system control of food intake. Nature 404(6778):661–671
Könner AC, Klöckener T, Brüning JC (2009) Control of energy homeostasis by insulin and leptin: targeting the arcuate nucleus and beyond. Physiol Behav 97(5):632–638
Ketterer C, Tschritter O, Preissl H, Heni M, Häring HU, Fritsche A (2011) Insulin sensitivity of the human brain. Diabetes Res Clin Pract Suppl 1:S47–S51
Martin SJ, Clark RE (2007) The rodent hippocampus and spatial memory: from synapses to systems. Cell Mol Life Sci 64(4):401–431
Haj-ali V, Mohaddes G, Babri SH (2009) Intracerebroventricular insulin improves spatial learning and memory in male Wistar rats. Behav Neurosci 123(6):1309–1314
Park CR, Seeley RJ, Craft S, Woods SC (2000) Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol Behav 68(4):509–514
Wallum BJ, Taborsky GJ Jr, Porte D Jr, Figlewicz DP, Jacobson L, Beard JC, Ward WK, Dorsa D (1987) Cerebrospinal fluid insulin levels increase during intravenous insulin infusions in man. J Clin Endocrinol Metab 64(1):190–194
Kern W, Peters A, Fruehwald-Schultes B, Deininger E, Born J, Fehm HL (2001) Improving influence of insulin on cognitive functions in humans. Neuroendocrinology 74(4):270–280
Fruehwald-Schultes B, Kern W, Born J, Fehm HL, Peters A (2001) Hyperinsulinemia causes activation of the hypothalamus-pituitary-adrenal axis in humans. Int J Obes Relat Metab Disord 25(Suppl 1):S38–S40
Het S, Ramlow G, Wolf OT (2005) A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology 30(8):771–784
Balin BJ, Broadwell RD, Salcman M, el-Kalliny M (1986) Avenues for entry of peripherally administered protein to the central nervous system in mouse, rat, and squirrel monkey. J Comp Neurol 251(2):260–280
Frey WH II (1997) Method of administering neurologic agents to the brain. US Patent 5,624,898 filed 1989 and issued April 29, 1997
Frey WH II (1991) Neurologic agents for nasal administration to the brain. PCT International Patent WO91/07947 filed 1990 and issued June 13, 1991
Frey WH II (2001) Method for administering insulin to the brain. US Patent 6,313,093 B1 filed 1999 and issued November 6, 2001
Jogani V, Jinturkar K, Vyas T, Misra A (2008) Recent patents review on intranasal administration for CNS drug delivery. Recent Pat Drug Deliv Formul 2(1):25–40
Thorne RG, Pronk GJ, Padmanabhan V, Frey WH (2004) Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 127(2):481–496
Dhuria SV, Hanson LR, Frey WH 2nd (2010) Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci 99(4):1654–1673
Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL (2002) Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci 5(6):514–516
Kern W, Born J, Schreiber H, Fehm HL (1999) Central nervous system effects of intranasally administered insulin during euglycemia in men. Diabetes 48(3):557–563
Hallschmid M, Schultes B, Marshall L, Mölle M, Kern W, Bredthauer J, Fehm HL, Born J (2004) Transcortical direct current potential shift reflects immediate signaling of systemic insulin to the human brain. Diabetes 53(9):2202–2208
Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, Kern W (2004) Intranasal insulin improves memory in humans. Psychoneuroendocrinology 29(10):1326–1334
Hallschmid M, Benedict C, Schultes B, Born J, Kern W (2008) Obese men respond to cognitive but not to catabolic brain insulin signaling. Int J Obes (Lond) 32(2):275–282
Benedict C, Hallschmid M, Schmitz K, Schultes B, Ratter F, Fehm HL, Born J, Kern W (2007) Intranasal insulin improves memory in humans: superiority of insulin aspart. Neuropsychopharmacology 32(1):239–243
Krug R, Benedict C, Born J, Hallschmid M (2010) Comparable sensitivity of postmenopausal and young women to the effects of intranasal insulin on food intake and working memory. J Clin Endocrinol Metab 95(12):E468–E472
Tan ZS, Beiser AS, Fox CS, Au R, Himali JJ, Debette S, Decarli C, Vasan RS, Wolf PA, Seshadri S (2011) Association of metabolic dysregulation with volumetric brain magnetic resonance imaging and cognitive markers of subclinical brain aging in middle-aged adults: the Framingham Offspring Study. Diabetes Care 34(8):1766–1770
Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K (2008) Central obesity and increased risk of dementia more than three decades later. Neurology 71:1057–1064
Tschritter O, Preissl H, Hennige AM, Stumvoll M, Porubska K, Frost R, Marx H, Klösel B, Lutzenberger W, Birbaumer N, Häring HU, Fritsche A (2006) The cerebrocortical response to hyperinsulinemia is reduced in overweight humans: a magnetoencephalographic study. Proc Natl Acad Sci U S A 103(32):12103–12108
Oakes TR, Pizzagalli DA, Hendrick AM, Horras KA, Larson CL, Abercrombie HC, Schaefer SM, Koger JV, Davidson RJ (2004) Functional coupling of simultaneous electrical and metabolic activity in the human brain. Hum Brain Mapp 21(4):257–270
Sauseng P, Klimesch W, Doppelmayr M, Hanslmayr S, Schabus M, Gruber WR (2004) Theta coupling in the human electroencephalogram during a working memory task. Neurosci Lett 354(2):123–126
Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S (2011) Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol 68(1):51–57
Bingham EM, Hopkins D, Smith D, Pernet A, Hallett W, Reed L, Marsden PK, Amiel SA (2002) The role of insulin in human brain glucose metabolism: an 18fluoro-deoxyglucose positron emission tomography study. Diabetes 51(12):3384–3390
Hoyer S (2002) The aging brain. Changes in the neuronal insulin/insulin receptor signal transduction cascade trigger late-onset sporadic Alzheimer disease (SAD). A mini-review. J Neural Transm 109(7–8):991–1002
Fujisawa Y, Sasaki K, Akiyama K (1991) Increased insulin levels after OGTT load in peripheral blood and cerebrospinal fluid of patients with dementia of Alzheimer type. Biol Psychiatry 30(12):1219–1228
Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D Jr (1999) Cerebrospinal fluid and plasma insulin levels in Alzheimer's disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology 50(1):164–168
Zhao WQ, Townsend M (2009) Insulin resistance and amyloidogenesis as common molecular foundation for type 2 diabetes and Alzheimer's disease. Biochim Biophys Acta 1792(5):482–496
Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM (2005) Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease—is this type 3 diabetes? J Alzheimers Dis 7(1):63–80
De Felice FG, Vieira MN, Bomfim TR, Decker H, Velasco PT, Lambert MP, Viola KL, Zhao WQ, Ferreira ST, Klein WL (2009) Protection of synapses against Alzheimer's-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci U S A 106(6):1971–1976
Hong M, Lee VM (1997) Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J Biol Chem 272(31):19547–19553
Francis G, Martinez J, Liu W, Nguyen T, Ayer A, Fine J, Zochodne D, Hanson LR, Frey WH 2nd, Toth C (2009) Intranasal insulin ameliorates experimental diabetic neuropathy. Diabetes 58(4):934–945
Craft S, Newcomer J, Kanne S, Dagogo-Jack S, Cryer P, Sheline Y, Luby J, Dagogo-Jack A, Alderson A (1996) Memory improvement following induced hyperinsulinemia in Alzheimer's disease. Neurobiol Aging 17(1):123–130
Reger MA, Watson GS, Frey WH 2nd, Baker LD, Cholerton B, Keeling ML, Belongia DA, Fishel MA, Plymate SR, Schellenberg GD, Cherrier MM, Craft S (2006) Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging 27(3):451–458
Cummings JL, Cole G (2002) Alzheimer disease. JAMA 287(18):2335–2338
Reger MA, Watson GS, Green PS, Baker LD, Cholerton B, Fishel MA, Plymate SR, Cherrier MM, Schellenberg GD, Frey WH 2nd, Craft S (2008) Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J Alzheimers Dis 13(3):323–331
Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JC, DeGroodt W, Mehta P, Craft S (2008) Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology 70(6):440–448
Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B (2011) Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. doi:10.1001/archneurol.2011.233
Craft S, Asthana S, Schellenberg G, Cherrier M, Baker LD, Newcomer J, Plymate S, Latendresse S, Petrova A, Raskind M, Peskind E, Lofgreen C, Grimwood K (1999) Insulin metabolism in Alzheimer's disease differs according to apolipoprotein E genotype and gender. Neuroendocrinology 70(2):146–152
Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, Fishel MA, Kulstad JJ, Green PS, Cook DG, Kahn SE, Keeling ML, Craft S (2005) Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry 13(11):950–958
Gold M, Alderton C, Zvartau-Hind M, Egginton S, Saunders AM, Irizarry M, Craft S, Landreth G, Linnamägi U, Sawchak S (2010) Rosiglitazone monotherapy in mild-to-moderate Alzheimer's disease: results from a randomized, double-blind, placebo-controlled phase III study. Dement Geriatr Cogn Disord 30(2):131–146
Fishel MA, Watson GS, Montine TJ, Wang Q, Green PS, Kulstad JJ, Cook DG, Peskind ER, Baker LD, Goldgaber D, Nie W, Asthana S, Plymate SR, Schwartz MW, Craft S (2005) Hyperinsulinemia provokes synchronous increases in central and peripheral inflammation and beta amyloid in normal older adults. Arch Neurol 62:1539–1544
Kern W, Peters A, Born J, Fehm HL, Schultes B (2005) Changes in blood pressure and plasma catecholamine levels during prolonged hyperinsulinemia. Metabolism 54(3):391–396
Benedict C, Dodt C, Hallschmid M, Lepiorz M, Fehm HL, Born J, Kern W (2005) Immediate but not long-term intranasal administration of insulin raises blood pressure in human beings. Metabolism 54(10):1356–1361
Taguchi A, Wartschow LM, White MF (2007) Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science 317(5836):369–372
Acknowledgments
Work from the authors' laboratories was supported by the Tore Nilsons Foundation, Ingrid Thurings Foundation, Brain Foundation, Åke Wiberg Foundation, Novo Nordisk, and the Swedish Research Council.
Disclosure Statement
The authors are unaware of any affiliation, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Schiöth, H.B., Craft, S., Brooks, S.J. et al. Brain Insulin Signaling and Alzheimer's Disease: Current Evidence and Future Directions. Mol Neurobiol 46, 4–10 (2012). https://doi.org/10.1007/s12035-011-8229-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-011-8229-6