Abstract
Background
Inflammasome-mediated neuroinflammation may cause secondary injury following traumatic brain injury (TBI) in children. The pattern recognition receptors NACHT domain-, Leucine-rich repeat-, and PYD-containing Protein 1 (NLRP1) and NLRP3 are essential components of their respective inflammasome complexes. We sought to investigate whether NLRP1 and/or NLRP3 abundance is altered in children with severe TBI.
Methods
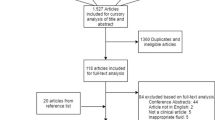
Cerebrospinal fluid (CSF) from children (n = 34) with severe TBI (Glasgow coma scale score [GCS] ≤8) who had externalized ventricular drains (EVD) placed for routine care was evaluated for NLRP1 and NLRP3 at 0–24, 25–48, 49–72, and >72 h post-TBI and was compared to infection-free controls that underwent lumbar puncture to rule out CNS infection (n = 8). Patient age, sex, initial GCS, mechanism of injury, treatment with therapeutic hypothermia, and 6-month Glasgow outcome score were collected.
Results
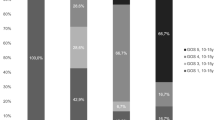
CSF NLRP1 was undetectable in controls and detected in 2 TBI patients at only <24 h post-TBI. CSF NLRP3 levels were increased in TBI patients compared with controls at all time points, p < 0.001. TBI patients ≤4 years of age had higher peak NLRP3 levels versus patients >4 (15.50 [3.65–25.71] vs. 3.04 [1.52–8.87] ng/mL, respectively; p = 0.048). Controlling for initial GCS in multivariate analysis, peak NLRP3 >6.63 ng/mL was independently associated with poor outcome at 6 months.
Conclusions
In the first report of NLRP1 and NLRP3 in childhood neurotrauma, we found that CSF NLRP3 is elevated in children with severe TBI and independently associated with younger age and poor outcome. Future studies correlating NLRP3 with other markers of inflammation and response to therapy are warranted.



Similar content being viewed by others
References
Coronado VG, Xu L, Basavaraju SV, et al. Surveillance for traumatic brain injury-related deaths—United States, 1997–2007. MMWR Surveill Summ. 2011;60:1–32.
Moreau JF, Fink EL, Hartman ME, et al. Hospitalizations of children with neurologic disorders in the United States. Pediatr Crit Care Med. 2013;14:801–10.
Fink KB, Andrews LJ, Butler WE, et al. Reduction of post-traumatic brain injury and free radical production by inhibition of the caspase-1 cascade. Neuroscience. 1999;94:1213–8.
Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–26.
Adamczak S, Dale G, de Rivero Vaccari JP, Bullock MR, Dietrich WD, Keane RW. Inflammasome proteins in cerebrospinal fluid of brain-injured patients as biomarkers of functional outcome: clinical article. J Neurosurg. 2012;117:1119–25.
de Rivero Vaccari JP, Dietrich WD, Keane RW. Activation and regulation of cellular inflammasomes: gaps in our knowledge for central nervous system injury. J Cereb Blood Flow Metab. 2014;34:369–75.
de Rivero Vaccari JP, Lotocki G, Alonso OF, Bramlett HM, Dietrich WD, Keane RW. Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J Cereb Blood Flow Metab. 2009;29:1251–61.
Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411.
Liu HD, Li W, Chen ZR, et al. Expression of the NLRP3 inflammasome in cerebral cortex after traumatic brain injury in a rat model. Neurochem Res. 2013;38:2072–83.
Frugier T, Morganti-Kossmann MC, O’Reilly D, McLean CA. In situ detection of inflammatory mediators in post mortem human brain tissue after traumatic injury. J Neurotrauma. 2010;27:497–507.
Satchell MA, Lai Y, Kochanek PM, et al. Cytochrome c, a biomarker of apoptosis, is increased in cerebrospinal fluid from infants with inflicted brain injury from child abuse. J Cereb Blood Flow Metab. 2005;25:919–27.
Kochanek PM, Carney N, Adelson PD, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents–second edition. Pediatr Crit Care Med. 2012;13(Suppl 1):S1–82.
Adelson PD, Wisniewski SR, Beca J, et al. Comparison of hypothermia and normothermia after severe traumatic brain injury in children (Cool Kids): a phase 3, randomised controlled trial. Lancet Neurol. 2013;12:546–53.
Savage CD, Lopez-Castejon G, Denes A, Brough D. NLRP3-inflammasome activating DAMPs stimulate an inflammatory response in glia in the absence of priming which contributes to brain inflammation after injury. Front Immunol. 2012;3:288.
Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109.
Fink SL, Bergsbaken T, Cookson BT. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc Natl Acad Sci USA. 2008;105:4312–7.
Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–5.
de Rivero Vaccari JP, Brand F 3rd, Adamczak S, et al. Exosome-mediated inflammasome signaling after central nervous system injury. J Neurochem. 2016;136(Suppl 1):39–48.
Luerssen TG, Klauber MR, Marshall LF. Outcome from head injury related to patient’s age. A longitudinal prospective study of adult and pediatric head injury. J Neurosurg. 1988;68:409–16.
Christian CW, Block R, Committee on Child Abuse and Neglect, American Academy of Pediatrics. Abusive head trauma in infants and children. Pediatrics. 2009;123:1409–11.
Shein SL, Bell MJ, Kochanek PM, et al. Risk factors for mortality in children with abusive head trauma. J Pediatr. 2012;161(716–22):e1.
Kemp AM, Joshi AH, Mann M, et al. What are the clinical and radiological characteristics of spinal injuries from physical abuse: a systematic review. Arch Dis Child. 2010;95:355–60.
Newell E, Shellington DK, Simon DW, et al. Cerebrospinal fluid markers of macrophage and lymphocyte activation after traumatic brain injury in children. Pediatr Crit Care Med. 2015;16:549–57.
Shiozaki T, Hayakata T, Tasaki O, et al. Cerebrospinal fluid concentrations of anti-inflammatory mediators in early-phase severe traumatic brain injury. Shock. 2005;23:406–10.
Chiaretti A, Genovese O, Aloe L, et al. Interleukin 1beta and interleukin 6 relationship with paediatric head trauma severity and outcome. Childs Nerv Syst. 2005;21:185–93.
Helmy A, Carpenter KL, Menon DK, Pickard JD, Hutchinson PJ. The cytokine response to human traumatic brain injury: temporal profiles and evidence for cerebral parenchymal production. J Cereb Blood Flow Metab. 2011;31:658–70.
Hutchinson PJ, O’Connell MT, Rothwell NJ, et al. Inflammation in human brain injury: intracerebral concentrations of IL-1alpha, IL-1beta, and their endogenous inhibitor IL-1ra. J Neurotrauma. 2007;24:1545–57.
Perez-Barcena J, Ibanez J, Brell M, et al. Lack of correlation among intracerebral cytokines, intracranial pressure, and brain tissue oxygenation in patients with traumatic brain injury and diffuse lesions. Crit Care Med. 2011;39:533–40.
Tehranian R, Andell-Jonsson S, Beni SM, et al. Improved recovery and delayed cytokine induction after closed head injury in mice with central overexpression of the secreted isoform of the interleukin-1 receptor antagonist. J Neurotrauma. 2002;19:939–51.
Clausen F, Hanell A, Bjork M, et al. Neutralization of interleukin-1beta modifies the inflammatory response and improves histological and cognitive outcome following traumatic brain injury in mice. Eur J Neurosci. 2009;30:385–96.
Clausen F, Hanell A, Israelsson C, et al. Neutralization of interleukin-1beta reduces cerebral edema and tissue loss and improves late cognitive outcome following traumatic brain injury in mice. Eur J Neurosci. 2011;34:110–23.
Sifringer M, Stefovska V, Endesfelder S, et al. Activation of caspase-1 dependent interleukins in developmental brain trauma. Neurobiol Dis. 2007;25:614–22.
Yatsiv I, Morganti-Kossmann MC, Perez D, et al. Elevated intracranial IL-18 in humans and mice after traumatic brain injury and evidence of neuroprotective effects of IL-18-binding protein after experimental closed head injury. J Cereb Blood Flow Metab. 2002;22:971–8.
Schmidt OI, Morganti-Kossmann MC, Heyde CE, et al. Tumor necrosis factor-mediated inhibition of interleukin-18 in the brain: a clinical and experimental study in head-injured patients and in a murine model of closed head injury. J Neuroinflammation. 2004;1:13.
Helmy A, Guilfoyle MR, Carpenter KL, Pickard JD, Menon DK, Hutchinson PJ. Recombinant human interleukin-1 receptor antagonist in severe traumatic brain injury: a phase II randomized control trial. J Cereb Blood Flow Metab. 2014;34:845–51.
Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263–9.
Coll RC, Robertson AA, Chae JJ, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–55.
Lamkanfi M, Mueller JL, Vitari AC, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187:61–70.
Acknowledgements
NINDS Grants R01 NS38620 (RC) and U01 NS081041 (MB); NICHD T32 HD40686 (DS, JW).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Jessica S. Wallisch and Dennis W. Simon have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wallisch, J.S., Simon, D.W., Bayır, H. et al. Cerebrospinal Fluid NLRP3 is Increased After Severe Traumatic Brain Injury in Infants and Children. Neurocrit Care 27, 44–50 (2017). https://doi.org/10.1007/s12028-017-0378-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-017-0378-7