Abstract
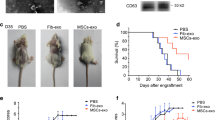
The mechanisms underlying immunomodulatory ability of mesenchymal stromal cells (MSCs) remain unknown. Recently, studies suggested that the immunomodulatory activity of MSCs is largely mediated by paracrine factors. Among which, exosome is considered to play a major role in the communication between MSCs and target tissue. The aim of our study is to investigate the effect of MSCs-derived exosome on peripheral blood mononuclear cells (PBMCs), especially T cells. We find that the MSCs-derived exosome extracted from healthy donors’ bone marrow suppressed the secretion of pro-inflammatory factor TNF-α and IL-1β, but increased the concentration of anti-inflammatory factor TGF-β during in vitro culture. In addition, exosome may induce conversion of T helper type 1 (Th1) into T helper type 2 (Th2) cells and reduced potential of T cells to differentiate into interleukin 17-producing effector T cells (Th17). Moreover, the level of regulatory T cells (Treg) and cytotoxic T lymphocyte-associated protein 4 were also increased. These results suggested that MSC-derived exosome possesses the immunomodulatory properties. However, it showed no effects on the proliferation of PBMCs or CD3+ T cells, but increases the apoptosis of them. In addition, indoleamine 2, 3-dioxygenase (IDO) was previously shown to mediate the immunoregulation of MSCs, which was increased in PBMCs co-cultured with MSCs. In our study, IDO showed no significant changes in PBMCs exposed to MSCs-derived exosome. We conclude that exosome and MSCs might differ in their immune-modulating activities and mechanisms.








Similar content being viewed by others
References
Nold P, et al. Immunosuppressive capabilities of mesenchymal stromal cells are maintained under hypoxic growth conditions and after gamma irradiation. Cytotherapy. 2015;17(2):152–62.
Tobin LM, et al. Human mesenchymal stem cells suppress donor CD4(+) T cell proliferation and reduce pathology in a humanized mouse model of acute graft-versus-host disease. Clin Exp Immunol. 2013;172(2):333–48.
Wuchter P, et al. Standardization of good manufacturing practice–compliant production of bone marrow–derived human mesenchymal stromal cells for immunotherapeutic applications. Cytotherapy. 2015;17(2):128–39.
Lee SM, Lee SC, Kim SJ. Contribution of human adipose tissue-derived stem cells and the secretome to the skin allograft survival in mice. J Surg Res. 2014;188(1):280–9.
Morigi M, Benigni A. Mesenchymal stem cells and kidney repair. Nephrol Dial Transplant. 2013;28(4):788–93.
Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–22.
Nauta AJ, et al. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177(4):2080–7.
Rakha A, Todeschini M, Casiraghi F. Assessment of anti-donor T cell proliferation and cytotoxic T lymphocyte-mediated lympholysis in living donor kidney transplant patients. Methods Mol Biol. 2014;1213:355–64.
Tabera S, et al. The effect of mesenchymal stem cells on the viability, proliferation and differentiation of B-lymphocytes. Haematologica. 2008;93(9):1301–9.
Swart J, et al. Mesenchymal stem cell therapy in proteoglycan induced arthritis. Ann Rheum Dis. 2015;74(4):769–77.
Woodworth TG, Furst DE. Safety and feasibility of umbilical cord mesenchymal stem cells in treatment-refractory systemic lupus erythematosus nephritis: time for a double-blind placebo-controlled trial to determine efficacy. Arthritis Res Ther. 2014;16(4):113.
Wu Y, et al. Cotransplantation of haploidentical hematopoietic and umbilical cord mesenchymal stem cells for severe aplastic anemia: successful engraftment and mild GVHD. Stem Cell Res. 2014;12(1):132–8.
Chinnadurai R, et al. Mesenchymal stromal cells derived from Crohn’s patients deploy indoleamine 2, 3-dioxygenase-mediated immune suppression, independent of autophagy. Mol Ther. 2015;23(7):1248–61.
de Mare-Bredemeijer EL, et al. Human graft-derived mesenchymal stromal cells potently suppress alloreactive T-cell responses. Stem Cells Dev. 2015;24(12):1436–47.
Laranjeira P, et al. Effect of human bone marrow mesenchymal stromal cells on cytokine production by peripheral blood naive, memory, and effector T cells. Stem Cell Res Ther. 2015;6(1):3.
Najar M, et al. Bone marrow mesenchymal stromal cells induce proliferative, cytokinic and molecular changes during the t cell response: the importance of the IL-10/CD210 axis. Stem Cell Rev Rep. 2015;11(3):442–52.
Akyurekli C, et al. A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell-derived microvesicles. Stem Cell Reviews and Reports. 2014;11(1):150–60.
Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195–208.
Bruno S, Camussi G. Exploring mesenchymal stem cell-derived extracellular vesicles in acute kidney injury. In: Animal models for stem cell therapy. Springer; 2014. p. 139–45.
Gouveia de Andrade AV, et al. Extracellular vesicles secreted by bone marrow-and adipose tissue-derived mesenchymal stromal cells fail to suppress lymphocyte proliferation. Stem Cells Dev. 2015;24(11):1374–6.
Katsuda T, et al. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics. 2013;13(10–11):1637–53.
Kordelas L, et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28(4):970.
Del Fattore A, et al. Immunoregulatory effects of mesenchymal stem cell-derived extracellular vesicles on T lymphocytes. Cell Transplant. 2015;24(12):2615–27.
Caby MP, et al. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17(7):879–87.
Hashemi SM, et al. Comparative immunomodulatory properties of adipose-derived mesenchymal stem cells conditioned media from BALB/c, C57BL/6, and DBA mouse strains. J Cell Biochem. 2013;114(4):955–65.
Castro-Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J Immunol Res. 2015;2015:394917.
Rosado MM, et al. Inhibition of B-cell proliferation and antibody production by mesenchymal stromal cells is mediated by T cells. Stem Cells Dev. 2015;24(1):93–103.
Ryan J, et al. Interferon-γ does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 2007;149(2):353–63.
Zhou Y, et al. Mesenchymal stromal cells augment CD4+ and CD8+ T-cell proliferation through a CCL2 pathway. Cytotherapy. 2013;15(10):1195–207.
Dorronsoro A, et al. Human mesenchymal stromal cells modulate T-cell responses through TNF-alpha-mediated activation of NF-kappaB. Eur J Immunol. 2014;44(2):480–8.
Silva A, et al. Bone marrow-derived mesenchymal stem cells and their conditioned medium attenuate fibrosis in an irreversible model of unilateral ureteral obstruction. Cell Transplant. 2015;24(12):2657–66.
Uchibori R, et al. Cancer gene therapy using mesenchymal stem cells. Int J Hematol. 2014;99(4):377–82.
Castro-Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J Immunol Res. 2015;2015:394917.
Conforti A, et al. Microvescicles derived from mesenchymal stromal cells are not as effective as their cellular counterpart in the ability to modulate immune responses in vitro. Stem cells and development. 2014;23(21):2591–9.
Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol. 2015;40:82–8.
Tan CY, Lai RC, Wong W, Dan YY, Lim S-K, Ho HK. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther. 2014;5:76.
Collino F, et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010;5(7):e11803.
Kilpinen L, et al. Extracellular membrane vesicles from umbilical cord blood-derived MSC protect against ischemic acute kidney injury, a feature that is lost after inflammatory conditioning. J Extracell Vesicles. 2013;2:21927.
Mokarizadeh A, et al. Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunol Lett. 2012;147(1):47–54.
Takahashi T, et al. Immunologic self-tolerance maintained by CD25(+) CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192(2):303–10.
Sivanathan KN, et al. Interleukin-17A-induced human mesenchymal stem cells are superior modulators of immunological function. Stem Cells. 2015;33(9):2850–63.
Acknowledgments
This study was supported by Important Special Subject Foundation of Guangzhou (201400000003-1; 201400000003-4) and Key Project of Natural Science Foundation of Guangdong Province (2014A0303110066), National Natural Science Foundation of China (81570107).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Additional information
Wancheng Chen and Yukai Huang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, W., Huang, Y., Han, J. et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol Res 64, 831–840 (2016). https://doi.org/10.1007/s12026-016-8798-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-016-8798-6