Abstract
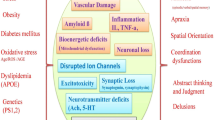
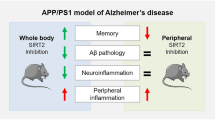
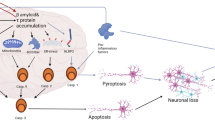
Aging is one of the major risk factors for Alzheimer’s disease (AD). Sirtuins are associated with prolonged life span. To examine whether the expression levels of sirtuins associate with the progression of AD or not, we performed a comparative immunoblotting and immunohistochemical study of SIRT1, 3, and 5 in the entorhinal cortex and hippocampal subregions and white matter in 45 cases grouped according to Braak and Braak stages of neurofibrillary degeneration. In addition, we compared the expression levels with the local load of tau and amyloid-beta deposits, evaluated using morphometry. Our study revealed that (1) the neuronal subcellular redistribution of SIRT1 parallels the decrease in its expression, suggesting stepwise loss of neuroprotection dependent on the neuronal population; (2) in contrast to SIRT1 and 3, expression of SIRT5 increases during the progression of AD; (3) which might be related to its appearance in activated microglial cells. The complex patterns of the expression of sirtuins in relation to tissue damage should be taken into account when searching for therapies interacting with sirtuins.


Similar content being viewed by others
References
Adori, C., Kovács, G. G., Low, P., et al. (2005). The ubiquitin-proteasome system in Creutzfeldt-Jakob and Alzheimer disease: Intracellular redistribution of components correlates with neuronal vulnerability. Neurobiol Dis., 19, 427–435.
Alzheimer A., Stelzmann R.A., Schnitzlein H.N., Murtagh F.R. (1995). An English translation of Alzheimer’s 1907 paper, Uber eine eigenartige Erkankung der Hirnrinde. Clin Anat (New York, NY) 8: 429–431.
Baur, J. A., Pearson, K. J., Price, N. L., et al. (2006). Resveratrol improves health and survival of mice on a high-calorie diet. Nature, 444, 337–342.
Boily, G., Seifert, E. L., Bevilacqua, L., et al. (2008). SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS One, 3, e1759.
Boyden, S. E., & Kunkel, L. M. (2010). High-density genomewide linkage analysis of exceptional human longevity identifies multiple novel loci. PLoS One, 5, e12432.
Braak, H., & Braak, E. (1991). Neuropathological stageing of Alzheimer-related changes. Acta Neuropatholog, 82, 239–259.
Brenmoehl, J., & Hoeflich, A. (2013). Dual control of mitochondrial biogenesis by sirtuin 1 and sirtuin 3. Mitochondrion. doi:10.1016/j.mito.2013.04.002.
Cai, D. (2013). Neuroinflammation and neurodegeneration in overnutrition-induced diseases. Trends Endocrinol Metabol TEM, 24, 40–47.
Cantó, C., & Auwerx, J. (2009). Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metabol TEM, 20, 325–331.
Chen, J., Zhou, Y., Mueller-Steiner, S., et al. (2005). SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem, 280, 40364–40374.
Costantini, S., Sharma, A., Raucci, R., et al. (2013). Genealogy of an ancient protein family: The Sirtuins, a family of disordered members. BMC Evol Biol, 13, 60.
Dai, H., Kustigian, L., Carney, D., et al. (2010). SIRT1 activation by small molecules: Kinetic and biophysical evidence for direct interaction of enzyme and activator. J Biol Chem, 285, 32695–32703.
De Luca, M., Rose, G., Bonafè, M., et al. (2001). Sex-specific longevity associations defined by Tyrosine Hydroxylase–Insulin–Insulin Growth Factor 2 haplotypes on the 11p15.5 chromosomal region. Exp Gerontol, 36, 1663–1671.
Dimauro, I., Pearson, T., Caporossi, D., & Jackson, M. J. (2012). A simple protocol for the subcellular fractionation of skeletal muscle cells and tissue. BMC Res Notes, 2012(5), 513.
Donmez, G., Wang, D., Cohen, D. E., & Guarente, L. (2010). SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell, 142, 320–332.
Du, J., Zhou, Y., Su, X., et al. (2011). Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science, 334, 806–809.
Duyckaerts, C., Delatour, B., & Potier, M. (2009). Classification and basic pathology of Alzheimer disease. Acta Neuropathol, 118, 5–36.
Gan, L., & Mucke, L. (2008). Paths of convergence: Sirtuins in aging and neurodegeneration. Neuron, 58, 10–14.
Geng, Y.-Q., Li, T–. T., Liu, X.-Y., et al. (2011). SIRT1 and SIRT5 activity expression and behavioral responses to calorie restriction. J Cell Biochem, 112, 3755–3761.
Glorioso, C., Oh, S., Douillard, G. G., & Sibille, E. (2011). Brain molecular aging, promotion of neurological disease and modulation by sirtuin 5 longevity gene polymorphism. Neurobiol Dis, 41, 279–290.
Goedert, M., & Spillantini, M. G. (2006). A century of Alzheimer’s disease. Science, 314, 777–781.
Hall, J. A., Dominy, J. E., Lee, Y., & Puigserver, P. (2013). The sirtuin family’s role in aging and age-associated pathologies. J Clin Invest, 123, 973–979.
Han, C., & Someya, S. (2013). Maintaining good hearing: Calorie restriction, Sirt3, and glutathione. Exp Gerontol. doi:10.1016/j.exger.2013.02.014.
Herskovits, A. Z., & Guarente, L. (2013). Sirtuin deacetylases in neurodegenerative diseases of aging. Cell Res, 23, 746–758.
Hisahara, S., Chiba, S., Matsumoto, H., et al. (2008). Histone deacetylase SIRT1 modulates neuronal differentiation by its nuclear translocation. Proc Natl Acad Sci USA, 105, 15599–15604.
Höftberger, R., Fink, S., Aboul-Enein, F., et al. (2010). Tubulin polymerization promoting protein (TPPP/p25) as a marker for oligodendroglial changes in multiple sclerosis. Glia, 58, 1847–1857.
Houtkooper, R. H., Pirinen, E., & Auwerx, J. (2012). Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol, 13, 225–238.
Howitz, K. T., Bitterman, K. J., Cohen, H. Y., et al. (2003). Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature, 425, 191–196.
Imai, S., & Guarente, L. (2010). Ten years of NAD-dependent SIR2 family deacetylases: Implications for metabolic diseases. Trends Pharmacol Sci, 31, 212–220.
Jin, Q., Yan, T., Ge, X., et al. (2007). Cytoplasm-localized SIRT1 enhances apoptosis. J Cell Physiol, 213, 88–97.
Julien, C., Tremblay, C., Emond, V., et al. (2009). Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J Neuropathol Exp Neurol, 68, 48–58.
Kim, D., Nguyen, M. D., Dobbin, M. M., et al. (2007). SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J, 26, 3169–3179.
Körner, S., Böselt, S., Thau, N., et al. (2013). Differential sirtuin expression patterns in amyotrophic lateral sclerosis (ALS) postmortem tissue: Neuroprotective or neurotoxic properties of Sirtuins in ALS? Neurodegener Dis, 11, 141–152.
Koubova, J., & Guarente, L. (2003). How does calorie restriction work? Genes Dev, 17, 313–321.
Kumar, R., Chaterjee, P., Sharma, P. K., et al. (2013). Sirtuin1: A promising serum protein marker for early detection of Alzheimer’s disease. PLoS One. doi:10.1371/journal.pone.0061560.
Li, X., Zhang, S., Blander, G., et al. (2007). SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell, 28, 91–106.
Luo, J., Nikolaev, A. Y., Imai, S., et al. (2001). Negative control of p53 by Sir2α promotes cell survival under stress. Cell, 107, 137–148.
Lynch, C. J., Shah, Z. H., Allison, S. J., et al. (2010). SIRT1 undergoes alternative splicing in a novel auto-regulatory loop with p53. PLoS One, 5, e13502.
Mahlknecht, U., Ho, A. D., Letzel, S., & Voelter-Mahlknecht, S. (2006). Assignment of the NAD-dependent deacetylase sirtuin 5 gene (SIRT5) to human chromosome band 6p23 by in situ hybridization. Cytogenet Genome Res, 112, 208–212.
Matsushita, N., Yonashiro, R., Ogata, Y., et al. (2011). Distinct regulation of mitochondrial localization and stability of two human Sirt5 isoforms. Genes cells, 16, 190–202.
Michishita, E., Park, J. Y., Burneskis, J. M., et al. (2005). Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell, 16, 4623–4635.
Min, S.-W., Cho, S.-H., Zhou, Y., et al. (2010). Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron, 67, 953–966.
Mirra, S. S., Heyman, A., McKeel, D., et al. (1991). The consortium to establish a registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology, 41, 479–486.
Misiak, B., Leszek, J., & Kiejna, A. (2012). Metabolic syndrome, mild cognitive impairment and Alzheimer’s disease–the emerging role of systemic low-grade inflammation and adiposity. Brain Res Bull, 89, 144–149.
Montine, T. J., Phelps, C. H., Beach, T. G., et al. (2012). National institute on Aging-Alzheimer’s association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol, 123, 1–11.
Mrak, R., & Griffin, W. (2005). Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging, 26, 349–354.
Onyango, P., Celic, I., McCaffery, J. M., et al. (2002). SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc Natl Acad Sci USA, 99, 13653–13658.
Oppenheimer, H., Gabay, O., Meir, H., et al. (2012). 75-kd sirtuin 1 blocks tumor necrosis factor α-mediated apoptosis in human osteoarthritic chondrocytes. Arthritis Rheum, 64, 718–728.
Panza, F., Frisardi, V., Capurso, C., et al. (2010). Metabolic syndrome and cognitive impairment: Current epidemiology and possible underlying mechanisms. J Alzheimer’s Dis, 21, 691–724.
Park, J. (2013). SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol Cell, 50, 919–930.
Peng, C., Lu, Z., Xie, Z., et al. (2011). The first identification of lysine malonylation substrates and its regulatory enzyme. Mol Cell Proteom, 10(M111), 012658.
Perry, V. H., Nicoll, J. A. R., & Holmes, C. (2010). Microglia in neurodegenerative disease. Nat Rev Neurol, 6, 193–201.
Polito, L., Kehoe, P. G., Forloni, G., & Albani, D. (2010). The molecular genetics of sirtuins: Association with human longevity and age-related diseases. Int J Mol Epidemiol Genetics, 1, 214–225.
Qin, W., Yang, T., Ho, L., et al. (2006). Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem, 281, 21745–21754.
Ramadori, G., Lee, C. E., Bookout, A. L., et al. (2008). Brain SIRT1: Anatomical distribution and regulation by energy availability. J Neurosci, 28, 9989–9996.
Rice, C. M., Sun, M., Kemp, K., et al. (2012). Mitochondrial sirtuins–a new therapeutic target for repair and protection in multiple sclerosis. Eur J Neurosci, 35, 1887–1893.
Rose, G., Dato, S., Altomare, K., et al. (2003). Variability of the SIRT3 gene, human silent information regulator Sir2 homologue, and survivorship in the elderly. Exp Gerontol, 38, 1065–1070.
Sakamoto, J., Miura, T., Shimamoto, K., & Horio, Y. (2004). Predominant expression of Sir2α, an NAD-dependent histone deacetylase, in the embryonic mouse heart and brain. FEBS Lett, 556, 281–286.
Schwer, B., North, B. J., Frye, R. A., et al. (2002). The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase. J Cell Biol, 158, 647–657.
Sugino, T., Maruyama, M., Tanno, M., et al. (2010). Protein deacetylase SIRT1 in the cytoplasm promotes nerve growth factor-induced neurite outgrowth in PC12 cells. FEBS Lett, 584, 2821–2826.
Tanno, M., Sakamoto, J., Miura, T., et al. (2007). Nucleocytoplasmic shuttling of the NAD + -dependent histone deacetylase SIRT1. J Biol Chem, 282, 6823–6832.
Thal, D. R., Rub, U., Orantes, M., & Braak, H. (2002). Phases of A-deposition in the human brain and its relevance for the development of AD. Neurology, 58, 1791–1800.
uniprot.com. (2013). NAD-dependent protein deacetylase sirtuin-1 - Homo sapiens (Human). http://www.uniprot.org/uniprot/Q96EB6#PRO_0000415289.
Vasiljevic, M., Heisler, F. F., Hausrat, T. J., et al. (2012). Spatio-temporal expression analysis of the calcium-binding protein calumenin in the rodent brain. Neuroscience, 202, 29–41.
Vaziri, H., Dessain, S. K., Eaton, E. N., et al. (2001). hSIR2SIRT1 Functions as an NAD-Dependent p53 Deacetylase. Cell, 107, 149–159.
Wood, J. G., Rogina, B., Lavu, S., et al. (2004). Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature, 430, 686–689.
Zhang, Z., Tan, M., Xie, Z., et al. (2011). Identification of lysine succinylation as a new post-translational modification. Nat Chem Biol, 7, 58–63.
Acknowledgments
The authors thank Irene Leisser, Gerda Ricken, and Eva Dassler for technical assistance and Dr. Thomas Ströbel for technical advice. This study was supported by DEVELAGE, a 7th framework program (FP7/2007-2013) under Grant Agreement No. 278486.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
12017_2014_8288_MOESM1_ESM.tif
Suppl.Fig. 1. Specificity control of SIRT1 and 5 antibodies. In comparison with the immunoreactivity of SIRT1 (a) and SIRT5 (c) antibody alone, the incubation with blocking peptide abolished immunolabeling in the tissue (b and d). (TIFF 6959 kb)
Rights and permissions
About this article
Cite this article
Lutz, M.I., Milenkovic, I., Regelsberger, G. et al. Distinct Patterns of Sirtuin Expression During Progression of Alzheimer’s Disease. Neuromol Med 16, 405–414 (2014). https://doi.org/10.1007/s12017-014-8288-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-014-8288-8