Abstract
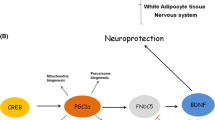
Brain-derived neurotrophic factor (BDNF) promotes the survival and growth of neurons during brain development and mediates activity-dependent synaptic plasticity and associated learning and memory in the adult. BDNF levels are reduced in brain regions affected in Alzheimer’s, Parkinson’s, and Huntington’s diseases, and elevation of BDNF levels can ameliorate neuronal dysfunction and degeneration in experimental models of these diseases. Because neurons accumulate oxidative lesions in their DNA during normal activity and in neurodegenerative disorders, we determined whether and how BDNF affects the ability of neurons to cope with oxidative DNA damage. We found that BDNF protects cerebral cortical neurons against oxidative DNA damage-induced death by a mechanism involving enhanced DNA repair. BDNF stimulates DNA repair by activating cyclic AMP response element-binding protein (CREB), which, in turn, induces the expression of apurinic/apyrimidinic endonuclease 1 (APE1), a key enzyme in the base excision DNA repair pathway. Suppression of either APE1 or TrkB by RNA interference abolishes the ability of BDNF to protect neurons against oxidized DNA damage-induced death. The ability of BDNF to activate CREB and upregulate APE1 expression is abolished by shRNA of TrkB as well as inhibitors of TrkB, PI3 kinase, and Akt kinase. Voluntary running wheel exercise significantly increases levels of BDNF, activates CREB, and upregulates APE1 in the cerebral cortex and hippocampus of mice, suggesting a novel mechanism whereby exercise may protect neurons from oxidative DNA damage. Our findings reveal a previously unknown ability of BDNF to enhance DNA repair by inducing the expression of the DNA repair enzyme APE1.






Similar content being viewed by others
References
Almeida, R. D., Manadas, B. J., Melo, C. V., Gomes, J. R., Mendes, C. S., Graos, M. M., et al. (2005). Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death and Differentiation, 12, 1329–1343.
Barnett, S. F., feo-Jones, D., Fu, S., Hancock, P. J., Haskell, K. M., Jones, R. E., et al. (2005). Identification and characterization of pleckstrin-homology-domain-dependent and isoenzyme-specific Akt inhibitors. Biochemical Journal, 385, 399–408.
Cazorla, M., Premont, J., Mann, A., Girard, N., Kellendonk, C., & Rognan, D. (2011). Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. The Journal of Clinical Investigation, 121, 1846–1857.
Cheng, A., Shin-ya, K., Wan, R., Tang, S. C., Miura, T., Tang, H., et al. (2007). Telomere protection mechanisms change during neurogenesis and neuronal maturation: Newly generated neurons are hypersensitive to telomere and DNA damage. Journal of Neuroscience, 27, 3722–3733.
Evans, A. R., Limp-Foster, M., & Kelley, M. R. (2000). Going APE over ref-1. Mutation Research, 461, 83–108.
Finkbeiner, S., Tavazoie, S. F., Maloratsky, A., Jacobs, K. M., Harris, K. M., & Greenberg, M. E. (1997). CREB: A major mediator of neuronal neurotrophin responses. Neuron, 19, 1031–1047.
Gilmore, E. C., Nowakowski, R. S., Caviness, V. S, Jr, & Herrup, K. (2000). Cell birth, cell death, cell diversity and DNA breaks: How do they all fit together? Trends in Neurosciences, 23, 100–105.
Gleichmann, M., Chow, V. W., & Mattson, M. P. (2011). Homeostatic disinhibition in the aging brain and Alzheimer’s disease. Journal of Alzheimer’s disease, 24, 15–24.
Gomez-Pinilla, F. (2008). The influences of diet and exercise on mental health through hormesis. Ageing Research Reviews, 7, 49–62.
Grosch, S., & Kaina, B. (1999). Transcriptional activation of apurinic/apyrimidinic endonuclease (Ape, Ref-1) by oxidative stress requires CREB. Biochemical and Biophysical Research Communications, 261, 859–863.
Han, B. H., & Holtzman, D. M. (2000). BDNF protects the neonatal brain from hypoxic-ischemic injury in vivo via the ERK pathway. Journal of Neuroscience, 20, 5775–5781.
Huang, E., Qu, D., Zhang, Y., Venderova, K., Haque, M. E., Rousseaux, M. W., et al. (2010). The role of Cdk5-mediated apurinic/apyrimidinic endonuclease 1 phosphorylation in neuronal death. Nature Cell Biology, 12, 563–571.
Kapogiannis, D., & Mattson, M. P. (2011). Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurology, 10, 187–198.
Kharebava, G., Makonchuk, D., Kalita, K. B., Zheng, J. J., & Hetman, M. (2008). Requirement of 3-phosphoinositide-dependent protein kinase-1 for BDNF-mediated neuronal survival. Journal of Neuroscience, 28, 11409–11420.
Khursigara, G., Bertin, J., Yano, H., Moffett, H., DiStefano, P. S., & Chao, M. V. (2001). A prosurvival function for the p75 receptor death domain mediated via the caspase recruitment domain receptor-interacting protein 2. Journal of Neuroscience, 21, 5854–5863.
Kulkarni, A., McNeill, D. R., Gleichmann, M., Mattson, M. P., & Wilson, D. M., III (2008). XRCC1 protects against the lethality of induced oxidative DNA damage in nondividing neural cells. Nucleic Acids Research, 36, 5111–5121.
Li, N., Wu, H., Yang, S., & Chen, D. (2007). Ischemic preconditioning induces XRCC1, DNA polymerase-beta, and DNA ligase III and correlates with enhanced base excision repair. DNA Repair (Amst), 6, 1297–1306.
Liu, D., Croteau, D. L., Souza-Pinto, N., Pitta, M., Tian, J., Wu, C., et al. (2011). Evidence that OGG1 glycosylase protects neurons against oxidative DNA damage and cell death under ischemic conditions. Journal of Cerebral Blood Flow and Metabolism, 31, 680–692.
Liu, F., Fu, Y., & Meyskens, F. L, Jr. (2009). MiTF regulates cellular response to reactive oxygen species through transcriptional regulation of APE-1/Ref-1. The Journal of Investigative Dermatology, 129, 422–431.
Lu, Y., Christian, K., & Lu, B. (2008). BDNF: A key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiology of Learning and Memory, 89, 312–323.
Lu, T., Pan, Y., Kao, S. Y., Li, C., Kohane, I., Chan, J., et al. (2004). Gene regulation and DNA damage in the ageing human brain. Nature, 429, 883–891.
Marini, A. M., Jiang, H., Pan, H., Wu, X., & Lipsky, R. H. (2008). Hormesis: A promising strategy to sustain endogenous neuronal survival pathways against neurodegenerative disorders. Ageing Research Reviews, 7, 21–33.
Marini, A. M., Jiang, X., Wu, X., Pan, H., Guo, Z., Mattson, M. P., et al. (2007). Preconditioning and neurotrophins: A model for brain adaptation to seizures, ischemia and other stressful stimuli. Amino Acids, 32, 299–304.
Martin, L. J., Liu, Z., Pipino, J., Chestnut, B., & Landek, M. A. (2009). Molecular regulation of DNA damage-induced apoptosis in neurons of cerebral cortex. Cerebral Cortex, 19, 1273–1293.
Mattson, M. P., Gleichmann, M., & Cheng, A. (2008). Mitochondria in neuroplasticity and neurological disorders. Neuron, 60, 748–766.
Mattson, M. P., Maudsley, S., & Martin, B. (2004). BDNF and 5-HT: A dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends in Neurosciences, 27, 589–594.
McIntyre, C. C., & Hahn, P. J. (2010). Network perspectives on the mechanisms of deep brain stimulation. Neurobiology of Diseases, 38, 329–337.
Nakamura, T., & Lipton, S. A. (2010). Preventing Ca2+-mediated nitrosative stress in neurodegenerative diseases: Possible pharmacological strategies. Cell Calcium, 47, 190–197.
Neeper, S. A., Gomez-Pinilla, F., Choi, J., & Cotman, C. W. (1996). Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Research, 726, 49–56.
Reichardt, L. F. (2006). Neurotrophin-regulated signalling pathways. Philosophical Transactions of the Royal Society of London. Series B, Biological sciences, 361, 1545–1564.
Roze, E., Saudou, F., & Caboche, J. (2008). Pathophysiology of Huntington’s disease: From huntingtin functions to potential treatments. Current Opinion in Neurology, 21, 497–503.
Rybnikova, E., Gluschenko, T., Tulkova, E., Churilova, A., Jaroshevich, O., Baranova, K., et al. (2008). Preconditioning induces prolonged expression of transcription factors pCREB and NF-κB in the neocortex of rats before and following severe hypobaric hypoxia. Journal of Neurochemistry, 106, 1450–1458.
Saha, T., Rih, J. K., Roy, R., Ballal, R., & Rosen, E. M. (2010). Transcriptional regulation of the base excision repair pathway by BRCA1. Journal of Biological Chemistry, 285, 19092–19105.
Sartori, C. R., Pelagio, F. C., Teixeira, S. A., Valentinuzzi, V. S., Nascimento, A. L., Rogerio, F., et al. (2009). Effects of voluntary running on spatial memory and mature brain-derived neurotrophic factor expression in mice hippocampus after status epilepticus. Behavioural Brain Research, 203, 165–172.
Schabitz, W. R., Schwab, S., Spranger, M., & Hacke, W. (1997). Intraventricular brain-derived neurotrophic factor reduces infarct size after focal cerebral ischemia in rats. Journal of Cerebral Blood Flow and Metabolism, 17, 500–506.
Shen, H., Tong, L., Balazs, R., & Cotman, C. W. (2001). Physical activity elicits sustained activation of the cyclic AMP response element-binding protein and mitogen-activated protein kinase in the rat hippocampus. Neuroscience, 107, 219–229.
Stetler, R. A., Gao, Y., Zukin, R. S., Vosler, P. S., Zhang, L., Zhang, F., et al. (2010). Apurinic/apyrimidinic endonuclease APE1 is required for PACAP-induced neuroprotection against global cerebral ischemia. Proceedings of the National Academy of Sciences of the United States of America, 107, 3204–3209.
Stranahan, A. M., Lee, K., Martin, B., Maudsley, S., Golden, E., Cutler, R. G., et al. (2009). Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus, 19, 951–961.
Terasaki, Y., Sasaki, T., Yagita, Y., Okazaki, S., Sugiyama, Y., Oyama, N., et al. (2010). Activation of NR2A receptors induces ischemic tolerance through CREB signaling. Journal of Cerebral Blood Flow and Metabolism, 30, 1441–1449.
Wang, S., Xing, Z., Vosler, P. S., Yin, H., Li, W., Zhang, F., et al. (2008). Cellular NAD replenishment confers marked neuroprotection against ischemic cell death: Role of enhanced DNA repair. Stroke, 39, 2587–2595.
Weissman, L., de Souza-Pinto, N. C., Mattson, M. P., & Bohr, V. A. (2009). DNA base excision repair activities in mouse models of Alzheimer’s disease. Neurobiology of Aging, 30, 2080–2081.
Weissman, L., Jo, D. G., Sorensen, M. M., de Souza-Pinto, N. C., Markesbery, W. R., Mattson, M. P., et al. (2007). Defective DNA base excision repair in brain from individuals with Alzheimer’s disease and amnestic mild cognitive impairment 5. Nucleic Acids Research, 35, 5545–5555.
Wilson, D. M., III, & Bohr, V. A. (2007). The mechanics of base excision repair, and its relationship to aging and disease. DNA Repair (Amst), 6, 544–559.
Wilson, D. M., III, & McNeill, D. R. (2007). Base excision repair and the central nervous system. Neuroscience, 145, 1187–1200.
Woods, J. A., Young, A. J., Gilmore, I. T., Morris, A., & Bilton, R. F. (1997). Measurement of menadione-mediated DNA damage in human lymphocytes using the comet assay. Free Radical Research, 26, 113–124.
Yang, J. L., Tadokoro, T., Keijzers, G., Mattson, M. P., & Bohr, V. A. (2010). Neurons efficiently repair glutamate-induced oxidative DNA damage by a process involving CREB-mediated up-regulation of apurinic endonuclease 1. Journal of Biological Chemistry, 285, 28191–28199.
Yang, J. L., Weissman, L., Bohr, V. A., & Mattson, M. P. (2008). Mitochondrial DNA damage and repair in neurodegenerative disorders. DNA Repair (Amst), 7, 1110–1120.
Zhao, Z., Leister, W. H., Robinson, R. G., Barnett, S. F., feo-Jones, D., Jones, R. E., et al. (2005). Discovery of 2,3,5-trisubstituted pyridine derivatives as potent Akt1 and Akt2 dual inhibitors. Bioorganic & Medicinal Chemistry Letters, 15, 905–909.
Zuccato, C., & Cattaneo, E. (2009). Brain-derived neurotrophic factor in neurodegenerative diseases. Nature Reviews Neurology, 5, 311–322.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health, Chang Gung Medical Foundation Grant No. CLRPG871342, CMRPG8A0781, CRPG8B0051, and research grants NSC 101-2320-B-182A-007- and NSC 102-2320-B-182A-012- from National Science Council Taiwan.
Conflict of interest
The authors declare that they have no conflicts of interest to report.
Author information
Authors and Affiliations
Corresponding author
Additional information
Vilhelm A. Bohr and Mark P. Mattson are senior authors and have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, JL., Lin, YT., Chuang, PC. et al. BDNF and Exercise Enhance Neuronal DNA Repair by Stimulating CREB-Mediated Production of Apurinic/Apyrimidinic Endonuclease 1. Neuromol Med 16, 161–174 (2014). https://doi.org/10.1007/s12017-013-8270-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-013-8270-x