Abstract
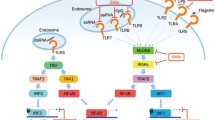
Autoimmune diseases are a family of chronic systemic inflammatory disorders, characterized by the dysregulation of the immune system which finally results in the break of tolerance to self-antigen. Several studies suggest that Toll-like receptors (TLRs) play an essential role in the pathogenesis of autoimmune diseases. TLRs belong to the family of pattern recognition receptors (PRRs) that recognize a wide range of pathogen-associated molecular patterns (PAMPs). TLRs are type I transmembrane proteins and located on various cellular membranes. Two main groups have been classified based on their location; the extracelluar group referred to the ones located on the plasma membrane while the intracellular group all located in endosomal compartments responsible for the recognition of nucleic acids. They are released by the host cells and trigger various intracellular pathways which results in the production of proinflammatory cytokines, chemokines, as well as the expression of co-stimulatory molecules to protect against invading microorganisms. In particular, TLR pathway-associated proteins, such as IRAK, TRAF, and SOCS, are often dysregulated in this group of diseases. TLR-associated gene expression profile analysis together with single nucleotide polymorphism (SNP) assessment could be important to explain the pathomechanism driving autoimmune diseases. In this review, we summarize recent findings on TLR pathway regulation in various autoimmune diseases, including Sjögren’s syndrome (SS), systemic lupus erythematosus (SLE), multiple sclerosis (MS), rheumatoid arthritis (RA), systemic sclerosis (SSc), and psoriasis.


Similar content being viewed by others
References
Amital H, Govoni M, Maya R et al (2008) Role of infectious agents in systemic rheumatic diseases. Clin Exp Rheumatol 26:S27–32
Liu Y, Yin H, Zhao M, Lu Q (2014) TLR2 and TLR4 in autoimmune diseases: a comprehensive review. Clin Rev Allergy Immunol 47:136–147. doi:10.1007/s12016-013-8402-y
Thwaites R, Chamberlain G, Sacre S (2014) Emerging role of endosomal toll-like receptors in rheumatoid arthritis. Front Immunol 5:1. doi:10.3389/fimmu.2014.00001
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124:783–801. doi:10.1016/j.cell.2006.02.015
Santegoets KCM, van Bon L, van den Berg WB et al (2011) Toll-like receptors in rheumatic diseases: are we paying a high price for our defense against bugs? FEBS Lett 585:3660–3666. doi:10.1016/j.febslet.2011.04.028
Mogensen TH, Paludan SR (2005) Reading the viral signature by Toll-like receptors and other pattern recognition receptors. J Mol Med Berl Ger 83:180–192. doi:10.1007/s00109-004-0620-6
Akira S, Takeda K (2004) Toll-like receptor signalling. Nat Rev Immunol 4:499–511. doi:10.1038/nri1391
Bowie A, O’Neill LA (2000) The interleukin-1 receptor/Toll-like receptor superfamily: signal generators for pro-inflammatory interleukins and microbial products. J Leukoc Biol 67:508–514
Kawai T, Akira S (2011) Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34:637–650. doi:10.1016/j.immuni.2011.05.006
Roach JC, Glusman G, Rowen L et al (2005) The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci U S A 102:9577–9582. doi:10.1073/pnas.0502272102
Takeda K, Akira S (2005) Toll-like receptors in innate immunity. Int Immunol 17:1–14. doi:10.1093/intimm/dxh186
Yamamoto M, Sato S, Mori K et al (2002) Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol 169:6668–6672
Yamamoto M, Sato S, Hemmi H et al (2003) Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301:640–643. doi:10.1126/science.1087262
Horng T, Barton GM, Flavell RA, Medzhitov R (2002) The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature 420:329–333. doi:10.1038/nature01180
Honda K, Yanai H, Mizutani T et al (2004) Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signaling. Proc Natl Acad Sci U S A 101:15416–15421. doi:10.1073/pnas.0406933101
Negishi H, Fujita Y, Yanai H et al (2006) Evidence for licensing of IFN-gamma-induced IFN regulatory factor 1 transcription factor by MyD88 in Toll-like receptor-dependent gene induction program. Proc Natl Acad Sci U S A 103:15136–15141. doi:10.1073/pnas.0607181103
Takaoka A, Yanai H, Kondo S et al (2005) Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature 434:243–249. doi:10.1038/nature03308
Kagan JC, Su T, Horng T et al (2008) TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol 9:361–368. doi:10.1038/ni1569
Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11:373–384. doi:10.1038/ni.1863
Shimazu R, Akashi S, Ogata H et al (1999) MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med 189:1777–1782
Kurt-Jones EA, Popova L, Kwinn L et al (2000) Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol 1:398–401. doi:10.1038/80833
Cao Z, Henzel WJ, Gao X (1996) IRAK: a kinase associated with the interleukin-1 receptor. Science 271:1128–1131
Janssens S, Beyaert R (2003) Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol Cell 11:293–302
O’Neill LAJ, Bryant CE, Doyle SL (2009) Therapeutic targeting of Toll-like receptors for infectious and inflammatory diseases and cancer. Pharmacol Rev 61:177–197. doi:10.1124/pr.109.001073
Wang C, Deng L, Hong M et al (2001) TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412:346–351. doi:10.1038/35085597
Hsu H, Xiong J, Goeddel DV (1995) The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell 81:495–504
Youn J, Kim H-Y, Park JH et al (2002) Regulation of TNF-alpha-mediated hyperplasia through TNF receptors, TRAFs, and NF-kappaB in synoviocytes obtained from patients with rheumatoid arthritis. Immunol Lett 83:85–93
Rothe M, Sarma V, Dixit VM, Goeddel DV (1995) TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science 269:1424–1427
Ihnatko R, Kubes M (2007) TNF signaling: early events and phosphorylation. Gen Physiol Biophys 26:159–167
Chung JY, Lu M, Yin Q et al (2007) Molecular basis for the unique specificity of TRAF6. Adv Exp Med Biol 597:122–130. doi:10.1007/978-0-387-70630-6_10
Lee NK, Lee SY (2002) Modulation of life and death by the tumor necrosis factor receptor-associated factors (TRAFs). J Biochem Mol Biol 35:61–66
Bradley JR, Pober JS (2001) Tumor necrosis factor receptor-associated factors (TRAFs). Oncogene 20:6482–6491. doi:10.1038/sj.onc.1204788
Wu H, Arron JR (2003) TRAF6, a molecular bridge spanning adaptive immunity, innate immunity and osteoimmunology. Bioessays News Rev Mol Cell Dev Biol 25:1096–1105. doi:10.1002/bies.10352
Naito A, Azuma S, Tanaka S et al (1999) Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells Devoted Mol Cell Mech 4:353–362
Endo TA, Masuhara M, Yokouchi M et al (1997) A new protein containing an SH2 domain that inhibits JAK kinases. Nature 387:921–924. doi:10.1038/43213
Starr R, Willson TA, Viney EM et al (1997) A family of cytokine-inducible inhibitors of signalling. Nature 387:917–921. doi:10.1038/43206
Naka T, Narazaki M, Hirata M et al (1997) Structure and function of a new STAT-induced STAT inhibitor. Nature 387:924–929. doi:10.1038/43219
Li YC, Chen Y, Liu W, Thadhani R (2014) MicroRNA-mediated mechanism of vitamin D regulation of innate immune response. J Steroid Biochem Mol Biol 144(Pt A):81–86. doi:10.1016/j.jsbmb.2013.09.014
Mansell A, Smith R, Doyle SL et al (2006) Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat Immunol 7:148–155. doi:10.1038/ni1299
Zeher M, Szodoray P (2009) Sjögren’s syndrome and associated disorders. Transworld Research Network, Kerala
Szanto A, Szodoray P, Kiss E et al (2006) Clinical, serologic, and genetic profiles of patients with associated Sjögren’s syndrome and systemic lupus erythematosus. Hum Immunol 67:924–930. doi:10.1016/j.humimm.2006.06.006
Zheng L, Zhang Z, Yu C, Yang C (2010) Expression of Toll-like receptors 7, 8, and 9 in primary Sjögren’s syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 109:844–850. doi:10.1016/j.tripleo.2010.01.006
Spachidou MP, Bourazopoulou E, Maratheftis CI et al (2007) Expression of functional Toll-like receptors by salivary gland epithelial cells: increased mRNA expression in cells derived from patients with primary Sjögren’s syndrome. Clin Exp Immunol 147:497–503. doi:10.1111/j.1365-2249.2006.03311.x
Kawakami A, Nakashima K, Tamai M et al (2007) Toll-like receptor in salivary glands from patients with Sjögren’s syndrome: functional analysis by human salivary gland cell line. J Rheumatol 34:1019–1026
Kwok S-K, Cho M-L, Her Y-M et al (2012) TLR2 ligation induces the production of IL-23/IL-17 via IL-6, STAT3 and NF-kB pathway in patients with primary Sjogren’s syndrome. Arthritis Res Ther 14:R64. doi:10.1186/ar3780
Zilahi E, Tarr T, Papp G et al (2012) Increased microRNA-146a/b, TRAF6 gene and decreased IRAK1 gene expressions in the peripheral mononuclear cells of patients with Sjögren’s syndrome. Immunol Lett 141:165–168. doi:10.1016/j.imlet.2011.09.006
Bhaumik D, Scott GK, Schokrpur S et al (2008) Expression of microRNA-146 suppresses NF-kappaB activity with reduction of metastatic potential in breast cancer cells. Oncogene 27:5643–5647. doi:10.1038/onc.2008.171
Szodoray P, Gal I, Barath S et al (2008) Immunological alterations in newly diagnosed primary Sjögren’s syndrome characterized by skewed peripheral T-cell subsets and inflammatory cytokines. Scand J Rheumatol 37:205–212. doi:10.1080/03009740801910361
Rothfield N (1989) Clinical aspects and treatment of systemic lupus erythematosus. Curr Opin Rheumatol 1:327–331
Savarese E, Chae O, Trowitzsch S et al (2006) U1 small nuclear ribonucleoprotein immune complexes induce type I interferon in plasmacytoid dendritic cells through TLR7. Blood 107:3229–3234. doi:10.1182/blood-2005-07-2650
Vollmer J, Tluk S, Schmitz C et al (2005) Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J Exp Med 202:1575–1585. doi:10.1084/jem.20051696
Means TK, Latz E, Hayashi F et al (2005) Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest 115:407–417. doi:10.1172/JCI23025
Barrat FJ, Meeker T, Gregorio J et al (2005) Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med 202:1131–1139. doi:10.1084/jem.20050914
Bengtsson AA, Sturfelt G, Truedsson L et al (2000) Activation of type I interferon system in systemic lupus erythematosus correlates with disease activity but not with antiretroviral antibodies. Lupus 9:664–671
Baechler EC, Batliwalla FM, Karypis G et al (2003) Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A 100:2610–2615. doi:10.1073/pnas.0337679100
Honda K, Ohba Y, Yanai H et al (2005) Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature 434:1035–1040. doi:10.1038/nature03547
Napolitani G, Rinaldi A, Bertoni F et al (2005) Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol 6:769–776. doi:10.1038/ni1223
Dennehy KM, Willment JA, Williams DL, Brown GD (2009) Reciprocal regulation of IL-23 and IL-12 following co-activation of Dectin-1 and TLR signaling pathways. Eur J Immunol 39:1379–1386. doi:10.1002/eji.200838543
Lyn-Cook BD, Xie C, Oates J et al (2014) Increased expression of Toll-like receptors (TLRs) 7 and 9 and other cytokines in systemic lupus erythematosus (SLE) patients: ethnic differences and potential new targets for therapeutic drugs. Mol Immunol 61:38–43. doi:10.1016/j.molimm.2014.05.001
Castiblanco J, Varela D-C, Castaño-Rodríguez N et al (2008) TIRAP (MAL) S180L polymorphism is a common protective factor against developing tuberculosis and systemic lupus erythematosus. Infect Genet Evol J Mol Epidemiol Evol Genet Infect Dis 8:541–544. doi:10.1016/j.meegid.2008.03.001
Zhang J, Zhu Q, Meng F et al (2014) Association study of TLR-9 polymorphisms and systemic lupus erythematosus in northern Chinese Han population. Gene 533:385–388. doi:10.1016/j.gene.2013.08.051
Wang C-M, Chang S-W, Wu Y-JJ et al (2014) Genetic variations in Toll-like receptors (TLRs 3/7/8) are associated with systemic lupus erythematosus in a Taiwanese population. Sci Rep 4:3792. doi:10.1038/srep03792
Zhou X-J, Lv J-C, Cheng W-R et al (2010) Association of TLR9 gene polymorphisms with lupus nephritis in a Chinese Han population. Clin Exp Rheumatol 28:397–400
Laska MJ, Troldborg A, Hansen B et al (2014) Polymorphisms within Toll-like receptors are associated with systemic lupus erythematosus in a cohort of Danish females. Rheumatol Oxf Engl 53:48–55. doi:10.1093/rheumatology/ket316
Sánchez E, García-Bermúdez M, Jiménez-Alonso J et al (2012) Association study of IRAK-M and SIGIRR genes with SLE in a large European-descent population. Lupus 21:1166–1171. doi:10.1177/0961203312449494
Zhu L, Yang X, Chen W et al (2007) Decreased expressions of the TNF-alpha signaling adapters in peripheral blood mononuclear cells (PBMCs) are correlated with disease activity in patients with systemic lupus erythematosus. Clin Rheumatol 26:1481–1489. doi:10.1007/s10067-006-0531-8
Tsao J-T, Kuo C-C, Lin S-C (2008) The analysis of CIS, SOCS1, SOSC2 and SOCS3 transcript levels in peripheral blood mononuclear cells of systemic lupus erythematosus and rheumatoid arthritis patients. Clin Exp Med 8:179–185. doi:10.1007/s10238-008-0006-0
Li J, Zhao S, Yi M et al (2011) Activation of JAK-STAT1 signal transduction pathway in lesional skin and monocytes from patients with systemic lupus erythematosus. Zhong Nan Da Xue Xue Bao Yi Xue Ban 36:109–115. doi:10.3969/j.issn. 1672-7347.2011.02.003
Pierangeli SS, Vega‐Ostertag ME, Raschi E et al (2007) Toll‐like receptor and antiphospholipid mediated thrombosis: in vivo studies. Ann Rheum Dis 66:1327–1333. doi:10.1136/ard.2006.065037
Polman CH, Reingold SC, Edan G et al (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.”. Ann Neurol 58:840–846. doi:10.1002/ana.20703
Gveric D, Kaltschmidt C, Cuzner ML, Newcombe J (1998) Transcription factor NF-kappaB and inhibitor I kappaBalpha are localized in macrophages in active multiple sclerosis lesions. J Neuropathol Exp Neurol 57:168–178
Christophi GP, Panos M, Hudson CA et al (2009) Macrophages of multiple sclerosis patients display deficient SHP-1 expression and enhanced inflammatory phenotype. Lab Investig J Tech Methods Pathol 89:742–759. doi:10.1038/labinvest.2009.32
Reynolds JM, Martinez GJ, Chung Y, Dong C (2012) Toll-like receptor 4 signaling in T cells promotes autoimmune inflammation. Proc Natl Acad Sci U S A 109:13064–13069. doi:10.1073/pnas.1120585109
Rolls A, Shechter R, London A et al (2007) Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol 9:1081–1088. doi:10.1038/ncb1629
Kostjuk S, Loseva P, Chvartatskaya O et al (2012) Extracellular GC-rich DNA activates TLR9- and NF-kB-dependent signaling pathways in human adipose-derived mesenchymal stem cells (haMSCs). Expert Opin Biol Ther 12(Suppl 1):S99–111. doi:10.1517/14712598.2012.690028
Sloane JA, Batt C, Ma Y et al (2010) Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc Natl Acad Sci U S A 107:11555–11560. doi:10.1073/pnas.1006496107
Hanafy KA, Sloane JA (2011) Regulation of remyelination in multiple sclerosis. FEBS Lett 585:3821–3828. doi:10.1016/j.febslet.2011.03.048
Hirotani M, Niino M, Fukazawa T et al (2010) Decreased IL-10 production mediated by Toll-like receptor 9 in B cells in multiple sclerosis. J Neuroimmunol 221:95–100. doi:10.1016/j.jneuroim.2010.02.012
Liu Y-J (2005) IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol 23:275–306. doi:10.1146/annurev.immunol.23.021704.115633
Compton T, Kurt-Jones EA, Boehme KW et al (2003) Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol 77:4588–4596
Dolganiuc A, Oak S, Kodys K et al (2004) Hepatitis C core and nonstructural 3 proteins trigger toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology 127:1513–1524
Bieback K, Lien E, Klagge IM et al (2002) Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J Virol 76:8729–8736
Rizzo R, Gentili V, Casetta I et al (2012) Altered natural killer cells’ response to herpes virus infection in multiple sclerosis involves KIR2DL2 expression. J Neuroimmunol 251:55–64. doi:10.1016/j.jneuroim.2012.07.004
Hernández-Pedro NY, Espinosa-Ramirez G, de la Cruz VP et al (2013) Initial immunopathogenesis of multiple sclerosis: innate immune response. Clin Dev Immunol 2013:413465. doi:10.1155/2013/413465
Giacomini E, Severa M, Rizzo F et al (2013) IFN-β therapy modulates B-cell and monocyte crosstalk via TLR7 in multiple sclerosis patients. Eur J Immunol 43:1963–1972. doi:10.1002/eji.201243212
Vandenbroeck K, Alvarez J, Swaminathan B et al (2012) A cytokine gene screen uncovers SOCS1 as genetic risk factor for multiple sclerosis. Genes Immunol 13:21–28. doi:10.1038/gene.2011.44
Baker BJ, Akhtar LN, Benveniste EN (2009) SOCS1 and SOCS3 in the control of CNS immunity. Trends Immunol 30:392–400. doi:10.1016/j.it.2009.07.001
Sedeño-Monge V, Arcega-Revilla R, Rojas-Morales E et al (2014) Quantitative analysis of the suppressors of cytokine signaling 1 and 3 in peripheral blood leukocytes of patients with multiple sclerosis. J Neuroimmunol 273:117–119. doi:10.1016/j.jneuroim.2014.05.013
Wesemann DR, Dong Y, O’Keefe GM et al (2002) Suppressor of cytokine signaling 1 inhibits cytokine induction of CD40 expression in macrophages. J Immunol 169:2354–2360
Frisullo G, Mirabella M, Angelucci F et al (2007) The effect of disease activity on leptin, leptin receptor and suppressor of cytokine signalling-3 expression in relapsing-remitting multiple sclerosis. J Neuroimmunol 192:174–183. doi:10.1016/j.jneuroim.2007.08.008
Matarese G, Carrieri PB, la Cava A et al (2005) Leptin increase in multiple sclerosis associates with reduced number of CD4(+)CD25+ regulatory T cells. Proc Natl Acad Sci U S A 102:5150–5155. doi:10.1073/pnas.0408995102
Bjørbaek C, Elmquist JK, Frantz JD et al (1998) Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell 1:619–625
Sha Y, Markovic-Plese S (2011) A role of IL-1R1 signaling in the differentiation of Th17 cells and the development of autoimmune diseases. Self/Nonself 2:35–42. doi:10.4161/self.2.1.15639
Ramgolam VS, Markovic-Plese S (2010) Interferon-beta inhibits Th17 cell differentiation in patients with multiple sclerosis. Endocr Metab Immune Disord Drug Targets 10:161–167
Zhang X, Jin J, Peng X et al (2008) Simvastatin inhibits IL-17 secretion by targeting multiple IL-17-regulatory cytokines and by inhibiting the expression of IL-17 transcription factor RORC in CD4+ lymphocytes. J Immunol 180:6988–6996
Chen Z, Laurence A, Kanno Y et al (2006) Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A 103:8137–8142. doi:10.1073/pnas.0600666103
Weber MS, Zamvil SS (2008) Statins and demyelination. Curr Top Microbiol Immunol 318:313–324
Mancuso R, Saresella M, Hernis A et al (2013) Torque teno virus (TTV) in multiple sclerosis patients with different patterns of disease. J Med Virol 85:2176–2183. doi:10.1002/jmv.23707
Gibofsky A (2012) Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis. Am J Manag Care 18:S295–302
Coenen MJH, Enevold C, Barrera P et al (2010) Genetic variants in toll-like receptors are not associated with rheumatoid arthritis susceptibility or anti-tumour necrosis factor treatment outcome. PLoS One 5:e14326. doi:10.1371/journal.pone.0014326
Jaen O, Petit-Teixeira E, Kirsten H et al (2009) No evidence of major effects in several Toll-like receptor gene polymorphisms in rheumatoid arthritis. Arthritis Res Ther 11:R5. doi:10.1186/ar2589
Etem EO, Elyas H, Ozgocmen S et al (2011) The investigation of toll-like receptor 3, 9 and 10 gene polymorphisms in Turkish rheumatoid arthritis patients. Rheumatol Int 31:1369–1374. doi:10.1007/s00296-010-1472-8
Enevold C, Radstake TRD, Coenen MJH et al (2010) Multiplex screening of 22 single-nucleotide polymorphisms in 7 Toll-like receptors: an association study in rheumatoid arthritis. J Rheumatol 37:905–910. doi:10.3899/jrheum.090775
Sacre SM, Andreakos E, Kiriakidis S et al (2007) The Toll-like receptor adaptor proteins MyD88 and Mal/TIRAP contribute to the inflammatory and destructive processes in a human model of rheumatoid arthritis. Am J Pathol 170:518–525. doi:10.2353/ajpath.2007.060657
Kim K-W, Cho M-L, Oh H-J et al (2009) TLR-3 enhances osteoclastogenesis through upregulation of RANKL expression from fibroblast-like synoviocytes in patients with rheumatoid arthritis. Immunol Lett 124:9–17. doi:10.1016/j.imlet.2009.02.006
Brentano F, Schorr O, Gay RE et al (2005) RNA released from necrotic synovial fluid cells activates rheumatoid arthritis synovial fibroblasts via Toll-like receptor 3. Arthritis Rheum 52:2656–2665. doi:10.1002/art.21273
Sacre SM, Lo A, Gregory B et al (2008) Inhibitors of TLR8 reduce TNF production from human rheumatoid synovial membrane cultures. J Immunol 181:8002–8009
Ospelt C, Brentano F, Rengel Y et al (2008) Overexpression of toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis: toll-like receptor expression in early and longstanding arthritis. Arthritis Rheum 58:3684–3692. doi:10.1002/art.24140
Roelofs MF, Wenink MH, Brentano F et al (2009) Type I interferons might form the link between Toll-like receptor (TLR) 3/7 and TLR4-mediated synovial inflammation in rheumatoid arthritis (RA). Ann Rheum Dis 68:1486–1493. doi:10.1136/ard.2007.086421
Tamaki Y, Takakubo Y, Hirayama T et al (2011) Expression of Toll-like receptors and their signaling pathways in rheumatoid synovitis. J Rheumatol 38:810–820. doi:10.3899/jrheum.100732
Radstake TRDJ, Roelofs MF, Jenniskens YM et al (2004) Expression of toll-like receptors 2 and 4 in rheumatoid synovial tissue and regulation by proinflammatory cytokines interleukin-12 and interleukin-18 via interferon-gamma. Arthritis Rheum 50:3856–3865. doi:10.1002/art.20678
Seibl R, Birchler T, Loeliger S et al (2003) Expression and regulation of Toll-like receptor 2 in rheumatoid arthritis synovium. Am J Pathol 162:1221–1227. doi:10.1016/S0002-9440(10)63918-1
Pierer M, Rethage J, Seibl R et al (2004) Chemokine secretion of rheumatoid arthritis synovial fibroblasts stimulated by Toll-like receptor 2 ligands. J Immunol 172:1256–1265
Roelofs MF, Joosten LA, Abdollahi-Roodsaz S et al (2005) The expression of toll-like receptors 3 and 7 in rheumatoid arthritis synovium is increased and costimulation of toll-like receptors 3, 4, and 7/8 results in synergistic cytokine production by dendritic cells. Arthritis Rheum 52:2313–2322. doi:10.1002/art.21278
Maksymowych W, Russell AS (1987) Antimalarials in rheumatology: efficacy and safety. Semin Arthritis Rheum 16:206–221
Khraishi MM, Singh G (1996) The role of anti-malarials in rheumatoid arthritis—the American experience. Lupus 5(Suppl 1):S41–44
Sanjuan MA, Rao N, Lai K-TA et al (2006) CpG-induced tyrosine phosphorylation occurs via a TLR9-independent mechanism and is required for cytokine secretion. J Cell Biol 172:1057–1068. doi:10.1083/jcb.200508058
Kuznik A, Bencina M, Svajger U et al (2011) Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol 186:4794–4804. doi:10.4049/jimmunol.1000702
Isomäki P, Alanärä T, Isohanni P et al (2007) The expression of SOCS is altered in rheumatoid arthritis. Rheumatol Oxf Engl 46:1538–1546. doi:10.1093/rheumatology/kem198
Raghav SK, Gupta B, Agrawal C et al (2006) Expression of TNF-alpha and related signaling molecules in the peripheral blood mononuclear cells of rheumatoid arthritis patients. Mediat Inflamm 2006:12682. doi:10.1155/MI/2006/12682
Potter C, Eyre S, Cope A et al (2007) Investigation of association between the TRAF family genes and RA susceptibility. Ann Rheum Dis 66:1322–1326. doi:10.1136/ard.2006.065706
Lee A, Qiao Y, Grigoriev G et al (2013) Tumor necrosis factor α induces sustained signaling and a prolonged and unremitting inflammatory response in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum 65:928–938. doi:10.1002/art.37853
Casale R, Buonocore M, Matucci-Cerinic M (1997) Systemic sclerosis (scleroderma): an integrated challenge in rehabilitation. Arch Phys Med Rehabil 78:767–773
van Bon L, Cossu M, Loof A et al (2014) Proteomic analysis of plasma identifies the Toll-like receptor agonists S100A8/A9 as a novel possible marker for systemic sclerosis phenotype. Ann Rheum Dis 73:1585–1589. doi:10.1136/annrheumdis-2013-205013
Broen JCA, Bossini-Castillo L, van Bon L et al (2012) A rare polymorphism in the gene for Toll-like receptor 2 is associated with systemic sclerosis phenotype and increases the production of inflammatory mediators. Arthritis Rheum 64:264–271. doi:10.1002/art.33325
York MR, Nagai T, Mangini AJ et al (2007) A macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and toll-like receptor agonists. Arthritis Rheum 56:1010–1020. doi:10.1002/art.22382
van Lieshout AWT, Vonk MC, Bredie SJH et al (2009) Enhanced interleukin-10 production by dendritic cells upon stimulation with Toll-like receptor 4 agonists in systemic sclerosis that is possibly implicated in CCL18 secretion. Scand J Rheumatol 38:282–290. doi:10.1080/03009740802572467
Fineschi S, Goffin L, Rezzonico R et al (2008) Antifibroblast antibodies in systemic sclerosis induce fibroblasts to produce profibrotic chemokines, with partial exploitation of toll-like receptor 4. Arthritis Rheum 58:3913–3923. doi:10.1002/art.24049
Farina A, Cirone M, York M et al (2014) Epstein-Barr virus infection induces aberrant TLR activation pathway and fibroblast-myofibroblast conversion in scleroderma. J Invest Dermatol 134:954–964. doi:10.1038/jid.2013.423
Visse R, Nagase H (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92:827–839. doi:10.1161/01.RES.0000070112.80711.3D
Woessner JF (1991) Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J Off Publ Fed Am Soc Exp Biol 5:2145–2154
Kikuchi K, Kubo M, Hoashi T, Tamaki K (2002) Decreased MMP-9 activity in the serum of patients with diffuse cutaneous systemic sclerosis. Clin Exp Dermatol 27:301–305
Montagnana M, Volpe A, Lippi G et al (2007) Relationship between matrix metalloproteinases/tissue inhibitors of matrix metalloproteinases systems and autoantibody patterns in systemic sclerosis. Clin Biochem 40:837–842. doi:10.1016/j.clinbiochem.2007.03.023
Kikuchi K, Kubo M, Sato S et al (1995) Serum tissue inhibitor of metalloproteinases in patients with systemic sclerosis. J Am Acad Dermatol 33:973–978
Iredale JP, Benyon RC, Arthur MJ et al (1996) Tissue inhibitor of metalloproteinase-1 messenger RNA expression is enhanced relative to interstitial collagenase messenger RNA in experimental liver injury and fibrosis. Hepatolgy 24:176–184. doi:10.1002/hep.510240129, Baltim Md
Ciechomska M, Huigens CA, Hügle T et al (2013) Toll-like receptor-mediated, enhanced production of profibrotic TIMP-1 in monocytes from patients with systemic sclerosis: role of serum factors. Ann Rheum Dis 72:1382–1389. doi:10.1136/annrheumdis-2012-201958
Christophers E (2001) Psoriasis–epidemiology and clinical spectrum. Clin Exp Dermatol 26:314–320
Baker BS, Ovigne J-M, Powles AV et al (2003) Normal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: modulation of TLR expression in chronic plaque psoriasis. Br J Dermatol 148:670–679
Seung NR, Park EJ, Kim CW et al (2007) Comparison of expression of heat-shock protein 60, Toll-like receptors 2 and 4, and T-cell receptor gammadelta in plaque and guttate psoriasis. J Cutan Pathol 34:903–911. doi:10.1111/j.1600-0560.2007.00756.x
Curry JL, Qin J-Z, Bonish B et al (2003) Innate immune-related receptors in normal and psoriatic skin. Arch Pathol Lab Med 127:178–186. doi:10.1043/0003-9985(2003)127<178:IIRRIN>2.0.CO;2
Gaspari AA (2006) Innate and adaptive immunity and the pathophysiology of psoriasis. J Am Acad Dermatol 54:S67–80. doi:10.1016/j.jaad.2005.10.057
Miller LS, Sørensen OE, Liu PT et al (2005) TGF-alpha regulates TLR expression and function on epidermal keratinocytes. J Immunol 174:6137–6143
Hüffmeier U, Uebe S, Ekici AB et al (2010) Common variants at TRAF3IP2 are associated with susceptibility to psoriatic arthritis and psoriasis. Nat Genet 42:996–999. doi:10.1038/ng.688
Böhm B, Burkhardt H, Uebe S et al (2012) Identification of low-frequency TRAF3IP2 coding variants in psoriatic arthritis patients and functional characterization. Arthritis Res Ther 14:R84. doi:10.1186/ar3807
Eriksen KW, Woetmann A, Skov L et al (2010) Deficient SOCS3 and SHP-1 expression in psoriatic T cells. J Invest Dermatol 130:1590–1597. doi:10.1038/jid.2010.6
Madonna S, Scarponi C, Pallotta S et al (2012) Anti-apoptotic effects of suppressor of cytokine signaling 3 and 1 in psoriasis. Cell Death Dis 3:e334. doi:10.1038/cddis.2012.69
Sonkoly E, Wei T, Janson PCJ et al (2007) MicroRNAs: novel regulators involved in the pathogenesis of psoriasis? PLoS One 2:e610. doi:10.1371/journal.pone.0000610
Federici M, Giustizieri ML, Scarponi C et al (2002) Impaired IFN-gamma-dependent inflammatory responses in human keratinocytes overexpressing the suppressor of cytokine signaling 1. J Immunol 169:434–442
Kubo M (2013) Therapeutic hope for psoriasis offered by SOCS (suppressor of cytokine signaling) mimetic peptide. Eur J Immunol 43:1702–1705. doi:10.1002/eji.201343748
Begon E, Michel L, Flageul B et al (2007) Expression, subcellular localization and cytokinic modulation of Toll-like receptors (TLRs) in normal human keratinocytes: TLR2 up-regulation in psoriatic skin. Eur J Dermatol EJD 17:497–506. doi:10.1684/ejd.2007.0264
Rappersberger K, Komar M, Ebelin M-E et al (2002) Pimecrolimus identifies a common genomic anti-inflammatory profile, is clinically highly effective in psoriasis and is well tolerated. J Invest Dermatol 119:876–887. doi:10.1046/j.1523-1747.2002.00694.x
Kang SSW, Kauls LS, Gaspari AA (2006) Toll-like receptors: applications to dermatologic disease. J Am Acad Dermatol 54:951–983. doi:10.1016/j.jaad.2005.05.004, quiz 983–986
Litjens NHR, Rademaker M, Ravensbergen B et al (2004) Monomethylfumarate affects polarization of monocyte-derived dendritic cells resulting in down-regulated Th1 lymphocyte responses. Eur J Immunol 34:565–575. doi:10.1002/eji.200324174
Acknowledgments
This work was supported by the Chinese Scholarship Council, the grants of the Hungarian National Scientific Research Fund (OTKA) and TÁMOP-4.2.2.A-11/1/KONV-2012-0023 project, which is co-financed by the European Union and European Social Fund.
Conflict of interest
No disclosure to report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, JQ., Szodoray, P. & Zeher, M. Toll-Like Receptor Pathways in Autoimmune Diseases. Clinic Rev Allerg Immunol 50, 1–17 (2016). https://doi.org/10.1007/s12016-015-8473-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-015-8473-z