Abstract
Mesenchymal stem cells (MSCs) are multipotent progenitors present in the bone marrow stroma and in subcutaneous abdominal fat, an abundant and easily accessible source of MSCs with the ability to differentiate along multiple lineage pathways. The stem cell-associated transcription co-factor Zinc Finger Protein 521 (ZNF521/zfp521) has been implicated in the control of the homeostasis of hematopoietic, neural and osteo-adipogenic progenitors. Here we document through the analysis of a panel of human adipose-derived stem cells (hADSCs), that ZNF521 strongly inhibits the generation of mature adipocytes. Enforced overexpression of ZNF521 in these cells resulted in a significant delay and reduction in adipocyte differentiation upon exposure to inducers of adipogenesis. Of particular relevance, ZNF521 was able to inhibit the expression of ZNF423, recently identified as an essential commitment factor necessary for the generation of pre-adipocytes. Conversely, silencing of ZNF521 was found to significantly enhance the adipogenic differentiation of hADSCs. Inhibition of adipogenesis by ZNF521 was at least in part due to inhibition of EBF1. Taken together, these results confirm a role for ZNF521 as a key negative regulator of adipocyte differentiation of hADSCs.






Similar content being viewed by others
Abbreviations
- ZNF521:
-
Zinc finger protein 521
- MSCs:
-
Mesenchymal Stem Cells
- hADSCs:
-
human Adipose-Derived Stem Cells
- ZNF423:
-
Zinc finger protein 423
- EBF-1:
-
Early B Cell Factor-1
- PPARγ :
-
Peroxisome Proliferator-Activated Receptor gamma
- C/EBPs :
-
CAATT/enhancer binding proteins
- EBF-2:
-
Early B Cell Factor-2
- ZNF638:
-
Zinc finger protein 638
- NF1 :
-
Neurofibromin 1
- ADD1:
-
Adipocyte Determination and Differentiation-dependent factor 1
- SREBP1:
-
Sterol Regulatory Element-binding Protein 1
- CREB:
-
Cyclic AMP Response Element-binding protein
- KLFs:
-
Kruppel-like Factors
- GATA:
-
GATA binding protein
- PREF-1:
-
Preadipocyte Factor-1
- SIRT1:
-
Histone deacetylase Sirtuin 1
- TAZ:
-
Transcriptional-coactivator with PDZ-binding motif
- Krox20:
-
EGR C2H2-type zinc-finger protein
- FABP4:
-
Fatty Acid Binding Protein 4
- FAS:
-
Fatty Acid Synthase
- LPL:
-
Lipoprotein Lipase
- SCD:
-
Stearyl-CoA-Desaturase
- GLUT-4:
-
Glucose transporter 4
- C2H2-ZF:
-
Cys2-His2 zinc fingers
- WISP2:
-
WNT1 Inducible Signaling Pathway Protein 2
- BMP4 :
-
Bone Morphogenetic Protein 4
- UBC:
-
Ubiquitin C
- IRES:
-
Intra-Ribosomal Entry Site
- eGFP:
-
Enhanced Green Fluorescent Protein
- LDs:
-
Lipid Droplets
- PPRE:
-
PPAR-Responsive Element
- ACOX1:
-
Acyl-CoA Oxidase 1
- DEX:
-
Dexamethasone
- IBTX:
-
Isobutyl-methylxanthine
- BmMSCs:
-
Bone marrow Mesenchymal Stem Cells
- Runx2 :
-
Runt-related transcription factor 2
- Smad:
-
Small mother against decapentaplegic
- NuRD:
-
Nucleosome Remodeling Deacetylase
- PDGFR-α:
-
Platelet-Derived Growth Factor Receptor –α
- HDAC:
-
Histone Deacetylase
- GAPDH:
-
Glyceraldehyde 3-Phosphate Dehydrogenase
References
Schaffler, A., & Buchler, C. (2007). Concise review: Adipose tissue-derived stromal cells-basic and clinical implications for novel cell-based therapies. Stem Cells, 25, 818–827.
Zhu, Z. H., Song, W. Q., Zhang, C. Q., & Yin, J. M. (2016). Dimethyloxaloylglycine increases bone repair capacity of adipose-derived stem cells in the treatment of osteonecrosis of the femoral head. Experimental and Therapeutic Medicine, 12, 2843–2850.
Bunnell, B. A., Flaat, M., Gagliardi, C., Patel, B., & Ripoll, C. (2008). Adipose-derived stem cells: Isolation, expansion and differentiation. Methods, 45, 115–120.
Lefterova, M. I., & Lazar, M. A. (2009). New developments in adipogenesis. Trends in Endocrinology and Metabolism, 20, 107–114.
Lee, J. E., & Ge, K. (2014). Transcriptional and epigenetic regulation of PPARgamma expression during adipogenesis. Cell & Bioscience. https://doi.org/10.1186/2045-3701-4-29.
Moseti, D., Regassa, A., & Kim, W. K. (2016). Molecular regulation of Adipogenesis and potential anti-Adipogenic bioactive molecules. International Journal of Molecular Sciences. https://doi.org/10.3390/ijms17010124.
Mueller, E. (2014). Understanding the variegation of fat: Novel regulators of adipocyte differentiation and fat tissue biology. Biochimica et Biophysica Acta, 1842, 352–357.
Siersbaek, M. S., Loft, A., Aagaard, M. M., et al. (2012). Genome-wide profiling of peroxisome proliferator-activated receptor gamma in primary epididymal, inguinal, and brown adipocytes reveals depot-selective binding correlated with gene expression. Molecular and Cellular Biology, 32, 3452–3463.
Cristancho, A. G., & Lazar, M. A. (2011). Forming functional fat: A growing understanding of adipocyte differentiation. Nature Reviews. Molecular Cell Biology, 12, 722–734.
Gustafson, B., Hedjazifar, S., Gogg, S., Hammarstedt, A., & Smith, U. (2015). Insulin resistance and impaired adipogenesis. Trends in Endocrinology and Metabolism, 26, 193–200.
Gupta, R. K., Arany, Z., Seale, P., et al. (2010). Transcriptional control of preadipocyte determination by Zfp423. Nature, 464, 619–623.
Hammarstedt, A., Hedjazifar, S., Jenndahl, L., et al. (2013). WISP2 regulates preadipocyte commitment and PPARgamma activation by BMP4. Proceedings of the National Academy of Sciences of the United States of America, 110, 2563–2568.
Bond, H. M., Mesuraca, M., Carbone, E., et al. (2004). Early hematopoietic zinc finger protein (EHZF), the human homolog to mouse Evi3, is highly expressed in primitive human hematopoietic cells. Blood, 103, 2062–2070.
Bond, H. M., Mesuraca, M., Amodio, N., et al. (2008). Early hematopoietic zinc finger protein-zinc finger protein 521: A candidate regulator of diverse immature cells. The International Journal of Biochemistry & Cell Biology, 40, 848–854.
Mega, T., Lupia, M., Amodio, N., et al. (2011). Zinc finger protein 521 antagonizes early B-cell factor 1 and modulates the B-lymphoid differentiation of primary hematopoietic progenitors. Cell Cycle, 10, 2129–2139.
Mesuraca, M., Chiarella, E., Scicchitano, S., et al. (2015). EBF1 Antagonists of Potential Relevance in B-Lymphoid Malignancies. Biomed Res Int, doi: https://doi.org/10.1155/2015/165238.
Yamasaki, N., Miyazaki, K., Nagamachi, A., et al. (2010). Identification of Zfp521/ZNF521 as a cooperative gene for E2A-HLF to develop acute B-lineage leukemia. Oncogene, 29, 1963–1975.
Salerno, L., Cosentino, C., Morrone, G., & Amato, F. (2015). Computational modeling of a transcriptional switch underlying B-lymphocyte lineage commitment of hematopoietic multipotent cells. PLoS One. https://doi.org/10.1371/journal.pone.0132208.
Hiratsuka, T., Takei, Y., Ohmori, R., et al. (2016). ZFP521 contributes to pre-B-cell lymphomagenesis through modulation of the pre-B-cell receptor signaling pathway. Oncogene, 35, 3227–3238.
Sera, Y., Yamasaki, N., Oda, H., et al. (2016). Identification of cooperative genes for E2A-PBX1 to develop acute lymphoblastic leukemia. Cancer Science, 107, 890–898.
Kamiya, D., Banno, S., Sasai, N., et al. (2011). Intrinsic transition of embryonic stem-cell differentiation into neural progenitors. Nature, 470, 503–509.
Shen, S., Pu, J., Lang, B., & McCaig, C. D. (2011). A zinc finger protein Zfp521 directs neural differentiation and beyond. Stem Cell Research. https://doi.org/10.1186/scrt61.
Shahbazi, E., Moradi, S., Nemati, S., et al. (2016). Conversion of human fibroblasts to stably self-renewing neural stem cells with a single zinc-finger transcription factor. Stem Cell Reports, 6, 539–551.
Spina, R., Filocamo, G., Iaccino, E., et al. (2013). Critical role of zinc finger protein 521 in the control of growth, clonogenicity and tumorigenic potential of medulloblastoma cells. Oncotarget, 4, 1280–1292.
Liu, T. M., & Lee, E. H. (2013). Transcriptional regulatory cascades in Runx2-dependent bone development. Tissue Engineering. Part B, Reviews, 19, 254–263.
Hesse, E., Saito, H., Kiviranta, R., et al. (2010). Zfp521 controls bone mass by HDAC3-dependent attenuation of Runx2 activity. The Journal of Cell Biology, 191, 1271–1283.
Wu, M., Hesse, E., Morvan, F., et al. (2009). Zfp521 antagonizes Runx2, delays osteoblast differentiation in vitro, and promotes bone formation in vivo. Bone, 44, 528–536.
Addison, W. N., Fu, M. M., Yang, H. X., et al. (2014). Direct transcriptional repression of Zfp423 by Zfp521 mediates a bone morphogenic protein-dependent osteoblast versus adipocyte lineage commitment switch. Molecular and Cellular Biology, 34, 3076–3085.
Correa, D., Hesse, E., Seriwatanachai, D., et al. (2010). Zfp521 is a target gene and key effector of parathyroid hormone-related peptide signaling in growth plate chondrocytes. Developmental Cell, 19, 533–546.
Mesuraca, M., Galasso, O., Guido, L., et al. (2014). Expression profiling and functional implications of a set of zinc finger proteins, ZNF423, ZNF470, ZNF521, and ZNF780B, in primary osteoarthritic articular chondrocytes. Mediators Inflamm., doi: https://doi.org/10.1155/2014/318793.
Kang, S., Akerblad, P., Kiviranta, R., Gupta, R. K., Kajimura, S., & Griffin, M. J. (2012). Regulation of early adipose commitment by Zfp521. PLoS Biology. https://doi.org/10.1371/journal.pbio.1001433.
Tseng, K. Y., & Lin, S. (2015). Zinc finger factor 521 enhances adipogenic differentiation of mouse multipotent cells and human bone marrow mesenchymal stem cells. Oncotarget, 6, 14874–14884.
Kiviranta, R., Yamana, K., Saito, H., et al. (2013). Coordinated transcriptional regulation of bone homeostasis by Ebf1 and Zfp521 in both mesenchymal and hematopoietic lineages. The Journal of Experimental Medicine, 210, 969–985.
Leuci, V., Gammaitoni, L., Capellero, S., et al. (2009). Efficient transcriptional targeting of human hematopoietic stem cells and blood cell lineages by lentiviral vectors containing the regulatory element of the Wiskott-Aldrich syndrome gene. Stem Cells, 27, 2815–2823.
Chiarella, E., Carrà, G., Scicchitano, S., et al. (2014). UMG Lenti: Novel lentiviral vectors for efficient transgene- and reporter gene expression in human early hematopoietic progenitors. PLoS One. https://doi.org/10.1371/journal.pone.0114795.
Akerblad, P., Lind, U., Liberg, D., Bamberg, K., & Sigvardsson, M. (2002). Early B-cell factor (O/E-1) is a promoter of adipogenesis and involved in control of genes important for terminal adipocyte differentiation. Molecular and Cellular Biology, 22, 8015–8025.
Hesslein, D. G., Fretz, J. A., Xi, Y., et al. (2009). Ebf1-dependent control of the osteoblast and adipocyte lineages. Bone, 44, 537–546.
Jimenez, M. A., Akerblad, P., Sigvardsson, M., & Rosen, E. D. (2007). Critical role for Ebf1 and Ebf2 in the adipogenic transcriptional cascade. Molecular and Cellular Biology, 27, 743–757.
Kao, C. H., Hsiang, C. Y., & Ho, T. Y. (2012). Assessment of chitosan-affected metabolic response by peroxisome proliferator-activated receptor bioluminescent imaging-guided transcriptomic analysis. PLoS One. https://doi.org/10.1371/journal.pone.0034969.
Gong, M., Antony, S., Sakurai, R., Liu, J., Iacovino, M., & Rehan, V. K. (2016). Bone marrow mesenchymal stem cells of the intrauterine growth-restricted rat offspring exhibit enhanced adipogenic phenotype. International Journal of Obesity, 40, 1768–1775.
Matsubara, E., Sakai, I., Yamanouchi, J., et al. (2009). The role of zinc finger protein 521/early hematopoietic zinc finger protein in erythroid cell differentiation. The Journal of Biological Chemistry, 284, 3480–3487.
Bernaudo, F., Monteleone, F., Mesuraca, M., et al. (2015). Validation of a novel shotgun proteomic workflow for the discovery of protein-protein interactions: Focus on ZNF521. Journal of Proteome Research, 14, 1888–1899.
Hesse, E., Kiviranta, R., Wu, M., et al. (2010). Zinc finger protein 521, a new player in bone formation. Annals of the New York Academy of Sciences, 1192, 32–37.
Sun, C., Berry, W. L., & Olson, L. E. (2017). PDGFRα controls the balance of stromal and adipogenic cells during adipose tissue organogenesis. Development, 144, 83–94.
Kilroy, G., Burk, D. H., & Floyd, Z. E. (2016). Siah protein mediates early events in commitment to an Adipogenic pathway. The Journal of Biological Chemistry, 291, 27289–27297.
La Rocca, R., Fulciniti, M., Lakshmikanth, T., et al. (2009). Early hematopoietic zinc finger protein prevents tumor cell recognition by natural killer cells. Journal of Immunology, 182, 4529–4537.
Misaggi, R., Di Sanzo, M., Cosentino, C., et al. (2014). Identification of H ferritin-dependent and independent genes in K562 differentiating cells by targeted gene silencing and expression profiling. Gene, 535, 327–335.
Codispoti, B., Rinaldo, N., Chiarella, E., et al. (2017). Recombinant TAT-BMI-1 fusion protein induces ex vivo expansion of human umbilical cord blood-derived hematopoietic stem cells. Oncotarget, 8, 43782–43798.
Acknowledgements
All authors are acknowledged for their contribution to the study.
Funding
This work was supported by funds from PON03PE_00009_2 ICaRe and PON01_02834 PROMETEO to GM. AA, GN, BC, VL, YM were supported by PhD Programme in Molecular and Translational Oncology and Innovative Surgical Medical Technologies. EC and SS were supported by fellowship to fund PON03PE_00009_2 ICaRe.
Author information
Authors and Affiliations
Contributions
GM and HMB conceived and designed the experiments; EC, AA, GN performed the experiments and SS, VL, YM, BC and AC analyzed the data; GM, HBM, EC, AA, MM, wrote the paper. MG, OG, GG helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Electronic supplementary material
Additional file 1A-1B
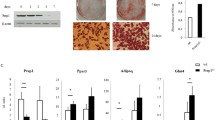
In a set of six different hADSCs experiments (n = 4) of ZNF521 overexpression and subsequent adipocyte differentiation the mRNA levels of the main adipocyte markers are consistantly reduced. (PNG 432 kb)
Additional file 1B
(PNG 178 kb)
Additional file 2A-2B
Six different experiments of ZNF521 silencing (n = 4) followed by adipocyte differentiation demonstrate a significant increase of the main genes involved during adipocyte differentiation. Only in one of these experiments (N°6) the shRNA-2 was inefficiently and consequently the induction of adipogenesis was only minimum. (PNG 1142 kb)
Additional file 2B
(PNG 367 kb)
Additional file 3
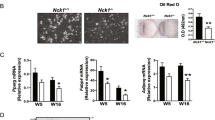
The ImageJ based programme used to process three images for each sample and to obtain the percentage of Oil red O stained area for field and the red intensity (pixels). The mean of percentage of stained area and the mean of absolute red intensity for each sample were compared to the corresponding controls both in stably overexpressing (Additional Fig. 3A) and silencing experiments (Additional Fig. 3B). (PNG 26 kb)
Rights and permissions
About this article
Cite this article
Chiarella, E., Aloisio, A., Codispoti, B. et al. ZNF521 Has an Inhibitory Effect on the Adipogenic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells. Stem Cell Rev and Rep 14, 901–914 (2018). https://doi.org/10.1007/s12015-018-9830-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-018-9830-0