Abstract
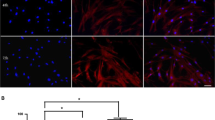
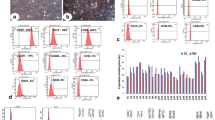
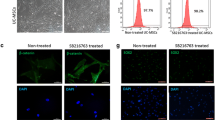
It was recently shown that the conditioned media (CM) of Human Umbilical Cord Perivascular Cells (HUCPVCs), a mesenchymal progenitor population residing within the Wharton Jelly of the umbilical cord, was able to modulate in vitro the survival and viability of different neuronal and glial cells populations. In the present work, we aimed to assess if the secretome of HUCPVCs is able to 1) induce the differentiation of human telencephalon neural precursor cells (htNPCs) in vitro, and 2) modulate neural/glial proliferation, differentiation and survival in the dentate gyrus (DG) of adult rat hippocampus. For this purpose, two separate experimental setups were performed: 1) htNPCs were incubated with HUCPVCs-CM for 5 days after which neuronal differentiation was assessed and, 2) HUCPVCs, or their respective CM, were injected into the DG of young adult rats and their effects assessed 7 days later. Results revealed that the secretome of HUCPVCs was able to increase neuronal cell differentiation in vitro; indeed, higher densities of immature (DCX+ cells) and mature neurons (MAP-2+ cells) were observed when htNPCs were incubated with the HUCPVCs-CM. Additionally, when HUCPVCs and their CM were injected in the DG, results revealed that both cells or CM were able to increase the endogenous proliferation (BrdU+ cells) 7 days after injection. It was also possible to observe an increased number of newborn neurons (DCX+ cells), upon injection of HUCPVCs or their respective CM. Finally western blot analysis revealed that after CM or HUCPVCs transplantation, there was an increase of fibroblast growth factor-2 (FGF-2) and, to a lesser extent, of nerve growth factor (NGF) in the DG tissue. Concluding, our results have shown that the transplantation of HUCPVCs or the administration of their secretome were able to potentiate neuronal survival and differentiation in vitro and in vivo.







Similar content being viewed by others
References
Balu, D. T., & Lucki, I. (2009). Adult hippocampal neurogenesis: regulation, functional implications, and contribution to disease pathology. Neuroscience and Biobehavioral Reviews, 33(3), 232–52.
Kazanis, I. (2009). The subependymal zone neurogenic niche: a beating heart in the centre of the brain: how plastic is adult neurogenesis? Opportunities for therapy and questions to be addressed. Brain, 132(Pt 11), 2909–21.
Kazanis, I., Belhad, I. A., Faissner, A., & Ffrench-Constant, C. (2007). The adult mouse subependymal zone regenerates efficiently in the absence of tenascin-C. Journal of Neuroscience, 27(51), 13991–6.
Kan, I., Barhum, Y., Melamed, E., & Offen, D. (2011). Mesenchymal stem cells stimulate endogenous neurogenesis in the subventricular zone of adult mice. Stem Cell Reviews, 7(2), 404–12.
Lindvall, O., & Kokaia, Z. (2010). Stem cells in human neurodegenerative disorders–time for clinical translation? Journal of Clinical Investigation, 120(1), 29–40.
Shihabuddin, L. S., & Aubert, I. (2010). Stem cell transplantation for neurometabolic and neurodegenerative diseases. Neuropharmacology, 58(6), 845–54.
Pittenger, M. F., Mackay, A. M., Beck, S. C., Jaiswai, R. K., Douglas, R., Mosca, J. D., et al. (1999). Multilineage potential of adult human mesenchymal stem cells. Science, 284(5411), 143–7.
Zuk, P. A., Zhu, M., Ashjian, P., De Ugarte, D. A., Huang, J. I., Mizuno, H., et al. (2002). Human adipose tissue is a source of multipotent stem cells. Molecular Biology of the Cell, 13(12), 4279–95.
Wang, H. S., Hung, S. C., Peng, S. T., Huang, C. C., Wei, H. M., Guo, Y. J., et al. (2004). Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells, 22(7), 1330–7.
Daher, S. R., Johnstone, B. H., Phinney, D. G., & March, K. L. (2004). Adipose stromal/stem cells: basic and translational advances: the IFATS collection. Stem Cells, 26(10), 2664–5.
Phinney, D. G., & Isakova, I. (2005). Plasticity and therapeutic potential of mesenchymal stem cells in the nervous system. Current Pharmaceutical Design, 11(10), 1255–65.
Prockop, D. J., Azizi, S. A., Colter, D., Digirolamo, C., Kopen, G., & Phinney, D. G. (2000). Potential use of stem cells from bone marrow to repair the extracellular matrix and the central nervous system. Biochemical Society Transactions, 28(4), 341–5.
Wei, X., Zhao, L., Zhong, J., Gu, H., Feng, D., Johnstone, B. H., et al. (2009). Adipose stromal cells-secreted neuroprotective media against neuronal apoptosis. Neuroscience Letters, 462(1), 76–9.
Honmou, O., Onodera, R., Sasaki, M., Waxman, S. G., & Kocsis, J. D. (2012). Mesenchymal stem cells: therapeutic outlook for stroke. Trends in Molecular Medicine, 18(5), 292–7.
Constantin, G., Marconi, S., Rossi, B., Angiari, S., Anghileri, E., Gini, B., et al. (2009). Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells, 27(10), 2624–35.
Cristofanilli, M., Harris, V. K., Zigelbaum, A., Goossens, A. M., Lu, A., Rosenthal, H., et al. (2011). Mesenchymal stem cells enhance the engraftment and myelinating ability of allogeneic oligodendrocyte progenitors in dysmyelinated mice. Stem Cells and Development, 20(12), 2065–76.
Cova, L., Armentero, M. T., Zennaro, E., Calzarossa, C., Bossolasco, P., Busca, G., et al. (2010). Multiple neurogenic and neurorescue effects of human mesenchymal stem cell after transplantation in an experimental model of Parkinson’s disease. Brain Research, 1311, 12–27.
Erba, P., Terenghi, G., & Kingham, P. J. (2010). Neural differentiation and therapeutic potential of adipose tissue derived stem cells. Current Stem Cell Research & Therapy, 5(2), 153–60.
Arboleda, D., Forostyak, S., Jendelova, P., Marekova, D., Amemori, T., Pivonkova, H., et al. (2011). Transplantation of predifferentiated adipose-derived stromal cells for the treatment of spinal cord injury. Cellular and Molecular Neurobiology, 31(7), 1113–22.
Park, J. H., Kim, D. Y., Sung, I. Y., Choi, G. H., Jeon, M. H., Kim, K. K., et al. (2012). Long-term results of spinal cord injury therapy using mesenchymal stem cells derived from bone marrow in humans. Neurosurgery, 70(5), 1238–47. discussion 1247.
Taghizadeh, R. R., Cetrulo, K. J., & Cetrulo, C. L. (2011). Wharton’s Jelly stem cells: future clinical applications. Placenta, 32(Suppl 4), S311–5.
Datta, I., Mishra, S., Mohanty, L., Pulikkot, S., & Joshi, P. G. (2011). Neuronal plasticity of human Wharton’s jelly mesenchymal stromal cells to the dopaminergic cell type compared with human bone marrow mesenchymal stromal cells. Cytotherapy, 13(8), 918–32.
Salgado, A. J., Fraga, J. S., Mesquita, A. R., Neves, N. M., Reis, R. L., & Sousa, N. (2010). Role of human umbilical cord mesenchymal progenitors conditioned media in neuronal/glial cell densities, viability, and proliferation. Stem Cells and Development, 19(7), 1067–74.
Weiss, M. L., & Troyer, D. L. (2006). Stem cells in the umbilical cord. Stem Cell Reviews, 2(2), 155–62.
Sarugaser, R., Ennis, J., Stanford, W. L., & Davies, J. E. (2009). Isolation, propagation, and characterization of human umbilical cord perivascular cells (HUCPVCs). Methods in Molecular Biology, 482, 269–79.
Baksh, D., Yao, R., & Tuan, R. S. (2007). Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells, 25(6), 1384–92.
Sarugaser, R., Lickorish, D., Baksh, D., Hosseini, M. M., & Davies, J. E. (2005). Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells, 23(2), 220–9.
Weiss, M. L., Medicetty, S., Bledsoe, A. R., Rachakatia, R. S., Choi, M., Merchav, S., et al. (2006). Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells, 24(3), 781–92.
Hirko, A. C., Dallasen, R., Jomura, S., & Xu, Y. (2008). Modulation of inflammatory responses after global ischemia by transplanted umbilical cord matrix stem cells. Stem Cells, 26(11), 2893–901.
Yang, C. C., Shih, Y. H., Ko, M. H., Hsu, S. Y., Cheng, H., & Fu, Y. S. (2008). Transplantation of human umbilical mesenchymal stem cells from Wharton’s jelly after complete transection of the rat spinal cord. PloS One, 3(10), e3336.
Zhang, L., Zhang, H. T., Hong, S. Q., Ma, X., Jiang, X. D., & Xu, R. X. (2009). Cografted Wharton’s jelly cells-derived neurospheres and BDNF promote functional recovery after rat spinal cord transection. Neurochemical Research, 34(11), 2030–9.
Carvalho, M. M., Teixeira, F. G., Reis, R. L., Sousa, N., & Salgado, A. J. (2011). Mesenchymal stem cells in the umbilical cord: phenotypic characterization, secretome and applications in central nervous system regenerative medicine. Current Stem Cell Research & Therapy, 6(3), 221–8.
Ribeiro, C. A., Fraga, J. S., Graos, M., Neves, N. M., Reis, R. L., Gimble, J. M., et al. (2012). The secretome of stem cells isolated from the adipose tissue and Wharton jelly acts differently on central nervous system derived cell populations. Stem Cell Research & Therapy, 3(3), 18.
Teixeira, F. G., Carvalho, M. M., Sousa, N., & Salgado, A. J. (2013). Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration? Cellular and Molecular Life Sciences, 70(20), 3871–82.
Koh, S. H., Kim, K. S., Choi, M. R., Jung, K. H., Park, K. S., Chai, Y. G., et al. (2008). Implantation of human umbilical cord-derived mesenchymal stem cells as a neuroprotective therapy for ischemic stroke in rats. Brain Research, 1229, 233–48.
Ding, D. C., Shyu, W. C., Chiang, M. F., Lin, S. Z., Chang, Y. C., Wang, H. J., et al. (2007). Enhancement of neuroplasticity through upregulation of beta1-integrin in human umbilical cord-derived stromal cell implanted stroke model. Neurobiology of Disease, 27(3), 339–53.
Fraga, J. S., Silva, N. A., Lourenço, A. S., Gonçalves, V., Neves, N. M., Reis, R. L., et al. (2013). Unveiling the effects of the secretome of mesenchymal progenitors from the umbilical cord in different neuronal cell populations. Biochimie, 95(12), 2297–303.
Baghbaderani, B. A., Mukhida, K., Sen, A., Kallos, M. S., Hong, M., Mendez, I., et al. (2010). Bioreactor expansion of human neural precursor cells in serum-free media retains neurogenic potential. Biotechnology and Bioengineering, 105(4), 823–33.
Mendez, I., Dagher, A., Hong, M., Gaudet, P., Weerasinghe, S., McAlister, V., et al. (2002). Simultaneous intrastriatal and intranigral fetal dopaminergic grafts in patients with Parkinson disease: a pilot study. Report of three cases. Journal of Neurosurgery, 96(3), 589–96.
Mendez, I., Sanchez-Pernaute, R., Cooper, O., Vinuela, A., Ferrari, D., Bjorklund, L., et al. (2005). Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson’s disease. Brain, 128(Pt 7), 1498–510.
Paxinos, G., & Watson, C. (2004). Rat brain in stereotaxic coordinates (5th ed.). San Diego: Academic.
Bernardo, M. E., Locatelli, F., & Fibbe, W. E. (2009). Mesenchymal stromal cells. Annals of the New York Academy of Sciences, 1176, 101–17.
Chen, Y., Shao, J. Z., Xiang, L. X., Dong, X. J., & Zhang, G. R. (2008). Mesenchymal stem cells: a promising candidate in regenerative medicine. International Journal of Biochemistry and Cell Biology, 40(5), 815–20.
Baer, P.C. and Geiger, H. (2012). Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem Cells International, p. 812693.
Huang, A. H., Snyder, B. R., Cheng, P. H., & Chan, A. W. (2008). Putative dental pulp-derived stem/stromal cells promote proliferation and differentiation of endogenous neural cells in the hippocampus of mice. Stem Cells, 26(10), 2654–63.
Sarugaser, R., Hanoun, L., Keating, A., Stanford, W. L., & Davies, E. (2009). Human mesenchymal stem cells self-renew and differentiate according to a deterministic hierarchy. PloS One, 4(8), e6498.
Yannarelli, G., Pacienza, N., Cuniberti, L., Medin, J., Davies, J., & Keating, A. (2013). Brief report: the potential role of epigenetics on multipotent cell differentiation capacity of mesenchymal stromal cells. Stem Cells, 31(1), 215–20.
Ribeiro, C. A., Salgado, A. J., Fraga, J. S., Silva, N. A., Reis, R. L., & Sousa, N. (2011). The secretome of bone marrow mesenchymal stem cells-conditioned media varies with time and drives a distinct effect on mature neurons and glial cells (primary cultures). Journal of Tissue Engineering and Regenerative Medicine, 5(8), 668–72.
Munoz, J. R., Stoutenger, B. R., Robinson, A. P., Spees, J. L., & Prockop, D. J. (2005). Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proceedings of the National Academy of Sciences of the United States of America, 102(50), 18171–6.
Tfilin, M., Sudai, E., Merenlender, A., Gispan, I., Yadid, G., & Turgeman, G. (2010). Mesenchymal stem cells increase hippocampal neurogenesis and counteract depressive-like behavior. Molecular Psychiatry, 15(12), 1164–75.
Frielingsdorf, H., Simpson, D., Thal, L. J., & Pizzo, D. P. (2007). Nerve growth factor promotes survival of new neurons in the adult hippocampus. Neurobiology of Disease, 26(1), 47–55.
Manni, L., Rocco, M. L., Bianchi, P., Soligo, M., Guaragna, M., Barbar, S. P., et al. (2013). Nerve growth factor: basic studies and possible therapeutic applications. Growth Factors, 31(4), 115–22.
Kurata, S., Goto, T., Gunjigake, K. K., Kataoka, S., Kuroishi, N. K., Ono, K., et al. (2013). Nerve growth factor involves mutual interaction between neurons and satellite glial cells in the rat trigeminal ganglion. Acta Histochem Cytochem, 46(2), 65–73.
Chen, J., Lee, C. T., Errico, S. L., Becker, K. G., & Freed, W. J. (2007). Increases in expression of 14-3-3 eta and 14-3-3 zeta transcripts during neuroprotection induced by delta9-tetrahydrocannabinol in AF5 cells. Journal of Neuroscience Research, 85(8), 1724–33.
Bonner, H. P., Concannon, C. G., Bonner, C., Woods, I., Ward, M. W., & Prehn, J. H. (2010). Differential expression patterns of Puma and Hsp70 following proteasomal stress in the hippocampus are key determinants of neuronal vulnerability. Journal of Neurochemistry, 114(2), 606–16.
Sakurai, M., Ayukawa, K., Setsuie, R., Nishikawa, K., Hara, Y., Ohashi, H., et al. (2006). Ubiquitin C-terminal hydrolase L1 regulates the morphology of neural progenitor cells and modulates their differentiation. Journal of Cell Science, 119(Pt 1), 162–71.
Acknowledgments
Foundation Calouste Gulbenkian for funds under the scope of the Gulbenkian Programme to Support Cutting Edge Research in Life Sciences; Portuguese Foundation for Science and Technology (FCT) for Ciência 2007 program and IF Development Grant (A.J. Salgado), and pre-doctoral fellowship to F.G. Teixeira (SFRH / BD / 69637 / 2010); John E. Davies for kindly providing the HUCPVCs used in this work.
Conflict of Interest
The author(s) declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Teixeira, F.G., Carvalho, M.M., Neves-Carvalho, A. et al. Secretome of Mesenchymal Progenitors from the Umbilical Cord Acts as Modulator of Neural/Glial Proliferation and Differentiation. Stem Cell Rev and Rep 11, 288–297 (2015). https://doi.org/10.1007/s12015-014-9576-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-014-9576-2