Abstract
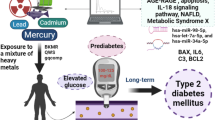
Accumulating studies have shown that chronic exposure to iAs correlates with an increased incidence of diabetes. In recent years, miRNA dysfunction has emerged both as a response to iAs exposure and independently as candidate drivers of metabolic phenotypes such as T2DM. However, few miRNAs have been profiled during the progression of diabetes after iAs exposure in vivo. In the present study, high iAs (10 mg/L NaAsO2) exposure mice models of C57BKS/Leprdb (db/db) and C57BLKS/J (WT) were established through the drinking water, the exposure duration was 14 weeks. The results showed that high iAs exposure induced no significant changes in FBG levels in either db/db or WT mice. FBI levels, C-peptide content, and HOMA-IR levels were significantly increased, and glycogen levels in the livers were significantly lower in arsenic-exposed db/db mice. HOMA-β% was decreased significantly in WT mice exposed to high iAs. In addition, more different metabolites were found in the arsenic-exposed group than the control group in db/db mice, mainly involved in the lipid metabolism pathway. Highly expressed glucose, insulin, and lipid metabolism-related miRNAs were selected, including miR-29a-3p, miR-143-3p, miR-181a-3p, miR-122-3p, miR-22-3p, and miR-16-3p. And a series of target genes were chosen for analysis, such as ptp1b, irs1, irs2, sirt1, g6pase, pepck and glut4. The results showed that, the axles of miR-181a-3p-irs2, miR-181a-3p-sirt1, miR-22-3p-sirt1, and miR-122-3p-ptp1b in db/db mice, and miR-22-3p-sirt1, miR-16-3p-glut4 in WT mice could be considered promising targets to explore the mechanisms and therapeutic aspects of T2DM after exposure to high iAs.






Similar content being viewed by others
Abbreviations
- T2DM:
-
Type 2 diabetes mellitus
- iAs:
-
Inorganic arsenic
- FBG:
-
Fasting blood glucose
- FBI:
-
Fasting blood insulin
- IR:
-
Insulin resistance
References
International Diabetes Federation (2019) Diab atlas ninth edition. Retrieved Nov 18, 2019, from https://www.diabetesatlas.org/en/
Paul DS, Harmon AW, Devesa V, Thomas DJ, Styblo M (2007) Molecular mechanisms of the diabetogenic effects of arsenic: inhibition of insulin signaling by arsenite and methylarsonous acid. Environ Health Perspect 115:734–742
Henning RJ (2018) Type-2 diabetes mellitus and cardiovascular disease. Futur Cardiol 14:491–509
Simon SL, Higgins J, Melanson E, Wright KP Jr, Nadeau KJ (2021) A model of adolescent sleep health and risk for type 2 diabetes. Curr Diab Rep 21:4
Takahashi K, Kamino T, Yasuda T, Suganuma A, Sakane N (2020) Association between psychological distress and stress-related symptoms and increased risk of type 2 diabetes in male individuals: An Observational Study. J Clin Med Res 12:816–823
Wabo TMC, Nkondjock VRN, Onwuka JU, Sun C, Han T, Sira J (2021) Association of fourteen years diet quality trajectories and type 2 diabetes mellitus with related biomarkers. Aging (Albany NY) 13:10112–10127
Sargis RM (2014) The hijacking of cellular signaling and the diabetes epidemic: mechanisms of environmental disruption of insulin action and glucose homeostasis. Diabetes Metab J 38:13–24
Mimoto MS, Nadal A, Sargis RM (2017) Polluted pathways: mechanisms of metabolic disruption by endocrine disrupting chemicals. Curr Environ Health Rep 4:208–222
Padmaja Divya S, Pratheeshkumar P, Son YO, Vinod Roy R, Andrew Hitron J, Kim D, Dai J, Wang L, Asha P, Huang B, Xu M, Luo J, Zhang Z (2015) Arsenic induces insulin resistance in mouse adipocytes and myotubes via oxidative stress-regulated mitochondrial Sirt3-FOXO3a Signaling Pathway. Toxicol Sci 146:290–300
Murcott S (2012) Arsenic contamination in the world. In: an International Sourcebook. UK (United Kindom): IWA Publishing
Navas-Acien A, Silbergeld EK, Streeter RA, Clark JM, Burke TA, Guallar E (2006) Arsenic exposure and type 2 diabetes: a systematic review of the experimental and epidemiological evidence. Environ Health Perspect 114:641–648
Islam R, Khan I, Hassan SN, McEvoy M, D'Este C, Attia J, Peel R, Sultana M, Akter S, Milton AH (2012) Association between type 2 diabetes and chronic arsenic exposure in drinking water: a cross sectional study in Bangladesh. Environ Health 11:38
Tseng CH, Tai TY, Chong CK, Tseng CP, Lai MS, Lin BJ, Chiou HY, Hsueh YM, Hsu KH, Chen CJ (2000) Long-term arsenic exposure and incidence of non-insulin-dependent diabetes mellitus: a cohort study in arseniasis-hyperendemic villages in Taiwan. Environ Health Perspect 108:847–851
Del Razo LM, Garcia-Vargas GG, Valenzuela OL, Castellanos EH, Sanchez-Pena LC, Currier JM, Drobna Z, Loomis D, Styblo M (2011) Exposure to arsenic in drinking water is associated with increased prevalence of diabetes: a cross-sectional study in the Zimapan and Lagunera regions in Mexico. Environ Health 10:73
Meliker JR, Wahl RL, Cameron LL, Nriagu JO (2007) Arsenic in drinking water and cerebrovascular disease, diabetes mellitus, and kidney disease in Michigan: a standardized mortality ratio analysis. Environ Health 6:4
Jovanovic D, Rasic-Milutinovic Z, Paunovic K, Jakovljevic B, Plavsic S, Milosevic J (2013) Low levels of arsenic in drinking water and type 2 diabetes in Middle Banat region. Serbia Int J Hyg Environ Health 216:50–55
Sturchio E, Colombo T, Boccia P, Carucci N, Meconi C, Minoia C, Macino G (2014) Arsenic exposure triggers a shift in microRNA expression. Sci Total Environ 472:672–680
Tan Y, Zhang B, Wu T, Skogerbø G, Zhu X, Guo X, He S, Chen R (2009) Transcriptional inhibiton of Hoxd4 expression by miRNA-10a in human breast cancer cells. BMC Mol Biol 10:12
He L, Hannon GJ (2004) MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 5:522–531
Kurtz CL, Fannin EE, Toth CL, Pearson DS, Vickers KC, Sethupathy P (2015) Inhibition of miR-29 has a significant lipid-lowering benefit through suppression of lipogenic programs in liver. Sci Rep 5:12911
Choi SE, Fu T, Seok S, Kim DH, Yu E, Lee KW, Kang Y, Li X, Kemper B, Kemper JK (2013) Elevated microRNA-34a in obesity reduces NAD+ levels and SIRT1 activity by directly targeting NAMPT. Aging Cell 12:1062–1072
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, Li Q, Li X, Wang W et al (2008) Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 18:997–1006
van de Bunt M, Gaulton KJ, Parts L, Moran I, Johnson PR, Lindgren CM, Ferrer J, Gloyn AL, McCarthy MI (2013) The miRNA profile of human pancreatic islets and beta-cells and relationship to type 2 diabetes pathogenesis. PLoS One 8:e55272
Huang W (2017) MicroRNAs: biomarkers, diagnostics, and therapeutics. Methods Mol Biol 1617:57–67
Filios SR, Shalev A (2015) beta-Cell MicroRNAs: Small but Powerful. Diabetes. 64:3631–3644
Sebastiani G, Nigi L, Grieco GE, Mancarella F, Ventriglia G, Dotta F (2017) Circulating microRNAs and diabetes mellitus: a novel tool for disease prediction, diagnosis, and staging? J Endocrinol Investig 40:591–610
Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E, Shah A, Willeit J, Mayr M (2010) Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res 107:810–817
Jordan SD, Kruger M, Willmes DM, Redemann N, Wunderlich FT, Bronneke HS, Merkwirth C, Kashkar H, Olkkonen VM, Bottger T, Braun T, Seibler J, Bruning JC (2011) Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism. Nat Cell Biol 13:434–446
1-Weksler-Zangen S, Yagil C, Zangen DH, Ornoy A, Jacob HJ, Yagil Y (2001) The newly inbred cohen diabetic rat: a nonobese normolipidemic genetic model of diet-induced type 2 diabetes expressing sex differences. Diabetes 50:2521–2529
Santos JM, Ribeiro SB, Gaya AR, Appell H-J, Duarte JA (2008) Skeletal muscle pathways of contraction-enhanced glucose uptake. Int J Sports Med 29:785–794
Delic D, Eisele C, Schmid R, Luippold G, Mayoux E, Grempler R (2016) Characterization of micro-RNA changes during the progression of type 2 diabetes in Zucker diabetic fatty rats. Int J Mol Sci 17(5):665
Wallace TM, Levy JC, Matthews DR (2004) Use and abuse of HOMA modeling. Diabetes Care 27:1487–1495
Maull EA, Ahsan H, Edwards J, Longnecker MP, Navas-Acien A, Pi J, Silbergeld EK, Styblo M, Tseng CH, Thayer KA, Loomis D (2012) Evaluation of the association between arsenic and diabetes: a National Toxicology Program workshop review. Environ Health Perspect 120:1658–1670
Navas-Acien A, Spratlen MJ, Abuawad A, LoIacono NJ, Bozack AK, Gamble MV (2019) Early-life arsenic exposure, nutritional status, and adult diabetes risk. Curr Diab Rep 19:147
Sawada N (2018) Association between arsenic intake and cancer-from the viewpoint of epidemiological study. Nihon Eiseigaku Zasshi 73:265–268
Liu S, Guo X, Wu B, Yu H, Zhang X, Li M (2014) Arsenic induces diabetic effects through beta-cell dysfunction and increased gluconeogenesis in mice. Sci Rep 4:6894
Wang-Sattler R, Yu Z, Herder C, Messias AC, Floegel A, He Y, Heim K, Campillos M, Holzapfel C, Thorand B, Grallert H, Xu T, Bader E, Huth C, Mittelstrass K, Doring A, Meisinger C, Gieger C, Prehn C et al (2012) Novel biomarkers for pre-diabetes identified by metabolomics. Mol Syst Biol 8:615
Floegel A, Stefan N, Yu Z, Muhlenbruch K, Drogan D, Joost HG, Fritsche A, Haring HU, Hrabe de Angelis M, Peters A, Roden M, Prehn C, Wang-Sattler R, Illig T, Schulze MB, Adamski J, Boeing H, Pischon T (2013) Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 62(2):639–648
Kougias P, Chai H, Lin PH, Lumsden AB, Yao Q, Chen C (2006) Lysophosphatidylcholine and secretory phospholipase A2 in vascular disease: mediators of endothelial dysfunction and atherosclerosis. Med Sci Monit 12(1):RA5–R16
Beck R, Styblo M, Sethupathy P (2017) Arsenic exposure and type 2 diabetes: MicroRNAs as mechanistic links? Curr Diab Rep 17(3):18
de Candia P, Spinetti G, Specchia C, Sangalli E, La Sala L, Uccellatore A, Lupini S, Genovese S, Matarese G, Ceriello A (2017) A unique plasma microRNA profile defines type 2 diabetes progression. PLoS One 12:e0188980
Willeit P, Skroblin P, Moschen AR, Yin X, Kaudewitz D, Zampetaki A, Barwari T, Whitehead M, Ramírez CM, Goedeke L, Rotllan N, Bonora E, Hughes AD et al (2017) Circulating MicroRNA-122 is associated with the risk of new-onset metabolic syndrome and type 2 diabetes. Diabetes 66:347–357
Gao W, He HW, Wang ZM, Zhao H, Lian XQ, Wang YS, Zhu J, Yan JJ, Zhang DG, Yang ZJ, Wang LS (2012) Plasma levels of lipometabolism-related miR-122 and miR-370 are increased in patients with hyperlipidemia and associated with coronary artery disease. Lipids Health Dis 11:55
Salvoza NC, Klinzing DC, Gopez-Cervantes J, Baclig MO (2016) Association of circulating serum miR-34a and miR-122 with dyslipidemia among patients with non-alcoholic fatty liver disease. PLoS One 11:e0153497
Koufaris C, Valbuena GN, Pomyen Y, Tredwell GD, Nevedomskaya E, Lau C-H, Yang T, Benito A, Ellis JK, Keun HC (2016) Systematic integration of molecular profiles identifies miR-22 as a regulator of lipid and folate metabolism in breast cancer cells. Oncogene 35(21):2766–2776
Kaur K, Vig S, Srivastava R et al (2015) Elevated hepatic miR-22-3p expression impairs gluconeogenesis by silencing the wnt-responsive transcription factor Tcf7. Diabetes 64(11):3659–3669
Li B, Fan J, Chen N (2018) A novel regulator of type II diabetes: MicroRNA-143. Trends Endocrinol Metab 29:380–388
Wu Y, Li XF, Yang JH, Liao XY, Chen YZ (2012) microRNAs expression profile in acute promyelocytic leukemia cell differentiation induced by all-trans retinoic acid and arsenic trioxide. Zhonghua Xue Ye Xue Za Zhi 33(7):546–551
Talari M, Kapadia B, Kain V et al (2015) MicroRNA-16 modulates macrophage polarization leading to improved insulin sensitivity in myoblasts. Biochimie 119:16–26
Todero JE, Koch-Laskowski K, Shi Q et al (2022) Candidate master microRNA regulator of arsenic-induced pancreatic beta cell impairment revealed by multi-omics analysis. Arch Toxicol. https://doi.org/10.1007/s00204-022-03263-9
Taniguchi CM, Emanuelli B, Kahn CR (2006) Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 7:85–96
Vivero A, Ruz M, Rivera M, Miranda K, Sacristán C, Espinosa A, Codoceo J, Inostroza J, Vásquez K, Pérez Á, García-Díaz D, Arredondo M (2021) Zinc supplementation and strength exercise in rats with type 2 diabetes: Akt and PTP1B phosphorylation in nonalcoholic fatty liver. Biol Trace Elem Res 199(6):2215–2224
Rai U, Kosuru R, Prakash S, Singh SP, Birla H, Tiwari V, Singh S (2019) Tetramethylpyrazine prevents diabetes by activating PI3K/Akt/GLUT-4 signalling in animal model of type-2 diabetes. Life Sci 236:116836
Sun Y, Liu S, Ferguson S, Wang L, Klepcyk P, Yun JS, Friedman JE (2002) Phosphoenolpyruvate carboxykinase overexpression selectively attenuates insulin signaling and hepatic insulin sensitivity in transgenic mice. J Biol Chem 277:23301–23307
Liang F, Kume S, Koya D (2009) SIRT1 and insulin resistance. Nat Rev Endocrinol 5:367–373
Zhang J (2007) The direct involvement of SirT1 in insulin-induced insulin receptor substrate-2 tyrosine phosphorylation. J Biol Chem 282:34356–34364
Renu K, Madhyastha H, Madhyastha R, Maruyama M, Arunachlam S, V GA. (2018) Role of arsenic exposure in adipose tissue dysfunction and its possible implication in diabetes pathophysiology. Toxicol Lett 284:86–95
Funding
This study was funded by The National Natural Science Foundation of China (Grant no. 81872562).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics Approval
All experimental procedures were approved by the Committee on Ethics of Animal Experiments of Harbin Medical University (hrbmuecdc20200323), and complied with the institutional Guide for the Care and Use of Laboratory Animals. And all authors confirmed that this study is reported in accordance with ARRIVE guidelines.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jackson Sira and Xiaodan Zhang contribute equally to this work.
Supplementary Information
ESM 1
(DOCX 14 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sira, J., Zhang, X., Gao, L. et al. Effects of Inorganic Arsenic on Type 2 Diabetes Mellitus In Vivo: the Roles and Mechanisms of miRNAs. Biol Trace Elem Res 202, 111–121 (2024). https://doi.org/10.1007/s12011-023-03669-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03669-1