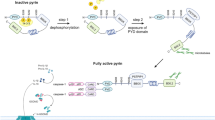
Abstract
Purpose of Review
Autoinflammatory diseases are driven by abnormal innate immune activation. In the case of inflammasomopathies, these are all attributable to activation of an inflammasome complex, nucleated by an innate immune sensor such as NLRP3. This review will focus on recent advances that have helped to elucidate the role of three other sensors (NLRP1, NLRC4 and pyrin) which can also cause inflammasomopathies.
Recent Findings
Mutations in pyrin (S242R or E244K) destroy an inhibitory 14-3-3 binding site and result in the newly characterised disease pyrin-associated autoinflammation with neutrophilic dermatosis (PAAND). Moreover, a separate autoinflammatory disease driven by mevalonate kinase deficiency leads to defective RhoGTPase prenylation and subsequent loss of pyrin S242R phosphorylation, suggesting a shared mechanism of disease. Other inflammasomes such as NLRP1 and NLRC4 have had novel mutations described recently, which inform about the specific domains required for activation and autoinhibition.
Summary
This review covers recent advances in the study of inflammasomopathies, focussing on gene discoveries that elucidate new pathogenic mechanisms.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–5.
Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535(7610):153–8.
Broderick L, de Nardo D, Franklin BS, Hoffman HM, Latz E. The Inflammasomes and autoinflammatory syndromes. Annu Rev Pathol. 2015;10:395–424.
Alghamdi M. Familial Mediterranean fever, review of the literature. Clin Rheumatol. 2017;36(8):1707–13.
• Masters SL, et al. Familial autoinflammation with neutrophilic dermatosis reveals a regulatory mechanism of pyrin activation. Science Translational Medicine. 2016;8(332):332ra45. In identifying a novel pyrin-driven autoinflammatory disease, PAAND, Masters et al. also demonstrated the role of 14-3-3 in pyrin regulation.
Moghaddas F, Llamas R, de Nardo D, Martinez-Banaclocha H, Martinez-Garcia JJ, Mesa-del-Castillo P, et al. A novel pyrin-associated autoinflammation with neutrophilic dermatosis mutation further defines 14-3-3 binding of pyrin and distinction to familial Mediterranean fever. Ann Rheum Dis. 2017;76(12):2085–94.
• Xu H, et al. Innate immune sensing of bacterial modifications of Rho GTPases by the pyrin inflammasome. Nature. 2014;513(7517):237–41. Xu et al. demonstrated that modification of RhoGTPases by pathogens leads to activation of the pyrin inflammasome.
Gao W, Yang J, Liu W, Wang Y, Shao F. Site-specific phosphorylation and microtubule dynamics control pyrin inflammasome activation. Proceedings of the National Academy of Sciences of the United States. 2016;113(33):E4857–66.
Park YH, Wood G, Kastner DL, Chae JJ. Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat Immunol. 2016;17(8):914–21.
Zhang S. Natural history of mevalonate kinase deficiency: a literature review. Pediatric Rheumatology Online Journal. 2016;14(1):30.
Seabra MC. Membrane association and targeting of prenylated Ras-like GTPases. Cell Signal. 1998;10(3):167–72.
Gorp HV, et al. Familial Mediterranean fever mutations lift the obligatory requirement for microtubules in pyrin inflammasome activation. Proceedings of the National Academy of Sciences of the United States. 2016;113(50):14384–9.
Kim ML, Chae JJ, Park YH, de Nardo D, Stirzaker RA, Ko HJ, et al. Aberrant actin depolymerization triggers the pyrin inflammasome and autoinflammatory disease that is dependent on IL-18, not IL-1β. J Exp Med. 2015;212(6):927–38.
Waite AL, et al. Pyrin and ASC co-localize to cellular sites that are rich in polymerizing actin. Exp Biol Med (Maywood). 2009;234(1):40–52.
Goldfinger S. Colchicine for familial Mediterranean fever. N Engl J Med. 1972;287(25):1302.
Lidar M, Scherrmann JM, Shinar Y, Chetrit A, Niel E, Gershoni-Baruch R, et al. Colchicine nonresponsiveness in familial Mediterranean fever: clinical, genetic, pharmacokinetic, and socioeconomic characterization. Semin Arthritis Rheum. 2004;33(4):273–82.
Kuhns DB, Fink DL, Choi U, Sweeney C, Lau K, Priel DL, et al. Cytoskeletal abnormalities and neutrophil dysfunction in WDR1 deficiency. Blood. 2016;128(17):2135–43.
Standing ASI, Malinova D, Hong Y, Record J, Moulding D, Blundell MP, et al. Autoinflammatory periodic fever, immunodeficiency, and thrombocytopenia (PFIT) caused by mutation in actin-regulatory gene WDR1. J Exp Med. 2017;214(1):59–71.
Kile BT, Panopoulos AD, Stirzaker RA, Hacking DF, Tahtamouni LH, Willson TA, et al. Mutations in the cofilin partner Aip1/Wdr1 cause autoinflammatory disease and macrothrombocytopenia. Blood. 2007;110(7):2371–80.
Gershoni-Baruch R, Shinawi M, Leah K, Badarnah K, Brik R. Familial Mediterranean fever: prevalence, penetrance and genetic drift. European Journal Of Human Genetics: EJHG. 2001;9(8):634–7.
Ratner D, et al. The Yersinia pestis effector YopM inhibits pyrin inflammasome activation. PLoS Pathog. 2016;12(12):1–17.
Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444(7117):323–9.
Grandemange S, Sanchez E, Louis-Plence P, Tran Mau-Them F, Bessis D, Coubes C, et al. A new autoinflammatory and autoimmune syndrome associated with NLRP1 mutations: NAIAD (NLRP1-associated autoinflammation with arthritis and dyskeratosis). Ann Rheum Dis. 2017;76(7):1191–8.
Finger JN, Lich JD, Dare LC, Cook MN, Brown KK, Duraiswami C, et al. Autolytic proteolysis within the function to find domain (FIIND) is required for NLRP1 inflammasome activity. J Biol Chem. 2012;287(30):25030–7.
• Zhong FL, et al. Germline NLRP1 mutations cause skin inflammatory and cancer susceptibility syndromes via inflammasome activation. Cell. 2016;167(1):187–202.e17. Zhong et al. describe two NLRP1-associated autoinflammatory diseases FKLC and MSPC. Furthermore, they demonstrate the that PYD domain of NLRP1 is autoinhibitory.
Soler VJ, Tran-Viet KN, Galiacy SD, Limviphuvadh V, Klemm TP, St Germain E, et al. Whole exome sequencing identifies a mutation for a novel form of corneal intraepithelial dyskeratosis. J Med Genet. 2013;50(4):246–54.
Mamaï O, Boussofara L, Denguezli M, Escande-Beillard N, Kraeim W, Merriman B, et al. Multiple self-healing palmoplantar carcinoma: a familial predisposition to skin cancer with primary palmoplantar and conjunctival lesions. J Investig Dermatol. 2015;135(1):304–8.
Adams S, et al. PT-100, a small molecule dipeptidyl peptidase inhibitor, has potent antitumor effects and augments antibody-mediated cytotoxicity via a novel immune mechanism. Cancer Res. 2004;64(15):5471–80.
Walsh MP, et al. Val-BoroPro accelerates T cell priming via modulation of dendritic cell trafficking resulting in complete regression of established murine tumors. PLoS One. 2013;8(3):1–13.
Okondo MC, et al. Inhibition of Dpp8/9 activates the Nlrp1b inflammasome. Cell Chem Biol. 2018;25:1-6.e1–5.
Okondo MC, Johnson DC, Sridharan R, Go EB, Chui AJ, Wang MS, et al. DPP8 and DPP9 inhibition induces pro-caspase-1-dependent monocyte and macrophage pyroptosis. Nat Chem Biol. 2016;13(1):46–53.
Kitamura A, Sasaki Y, Abe T, Kano H, Yasutomo K. An inherited mutation in NLRC4 causes autoinflammation in human and mice. J Exp Med. 2014;211(12):2385–96.
Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29(3):301–5.
Jéru I, et al. Mutations in NALP12 cause hereditary periodic fever syndromes. Proc Natl Acad Sci U S A. 2008;105(5):1614–9.
• Canna SW, et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nature Genetics. 2014;46(10):1140–6. Published back to back with Romberg et al. (2014); Canna et al. describe recurrent MAS with early-onset enterocolitis driven by NLRC4 gain-of-function mutations.
• Romberg N, et al. Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nature Genetics. 2014;46(10):1135–9. Published back to back with Canna et al (2014).
Nordlander S, Pott J, Maloy KJ. NLRC4 expression in intestinal epithelial cells mediates protection against an enteric pathogen. Mucosal Immunol. 2014;7(4):775–85.
Rauch I, Deets KA, Ji DX, von Moltke J, Tenthorey JL, Lee AY, et al. Article: NAIP-NLRC4 inflammasomes coordinate intestinal epithelial cell expulsion with eicosanoid and IL-18 release via activation of caspase-1 and -8. Immunity. 2017;46(4):649–59.
Hu B, Elinav E, Huber S, Booth CJ, Strowig T, Jin C, et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci. 2010;107(50):21635–40.
Sellin ME, Müller AA, Felmy B, Dolowschiak T, Diard M, Tardivel A, et al. Epithelium-intrinsic NAIP/NLRC4 inflammasome drives infected enterocyte expulsion to restrict Salmonella replication in the intestinal mucosa. Cell Host Microbe. 2014;16(2):237–48.
Scott W, Canna CG, Malle L, de Jesus A, Romberg N, Kelsen J, et al. Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. J Allergy Clin Immunol. 2017;139(5):1698–701.
Hu Z, Yan C, Liu P, Huang Z, Ma R, Zhang C, et al. Crystal structure of NLRC4 reveals its autoinhibition mechanism. Science. 2013;341(6142):172–5.
El-gharbawy A, et al. Novel NLRC4 mutation causes a syndrome of perinatal autoinflammation with hemophagocytic lymphohistiocytosis, hepatosplenomegaly, fetal thrombotic vasculopathy, and congenital anemia and ascites. Pediatr Dev Pathol. 2017;20(6):498–505.
Kawasaki Y, Oda H, Ito J, Niwa A, Tanaka T, Hijikata A, et al. Identification of a high-frequency somatic NLRC4 mutation as a cause of autoinflammation by pluripotent cell-based phenotype dissection. Arthritis Rheumatol. 2017;69(2):447–59.
Moghaddas F, Zeng P, Zhang Y, Schützle H, Brenner S, Hofmann SR, et al. Autoinflammatory mutation in NLRC4 reveals an LRR-LRR oligomerization interface. J Allergy Clin Immunol. 2018. https://doi.org/10.1016/j.jaci.2018.04.033.
Liston A, Masters SL. Homeostasis-altering molecular processes as mechanisms of inflammasome activation. Nat Rev Immunol. 2017;17(3):208–14.
Acknowledgements
The authors would like to thank members of the Masters Lab, particularly Dr. Paul Baker and Dr. Fiona Moghaddas, for discussion and advice on this review.
Funding
S.L.M acknowledges funding from NHMRC grants (1144282, 1142354 and 1099262), The Sylvia and Charles Viertel Foundation, HHMI-Wellcome International Research Scholarship and GlaxoSmithKline. SD acknowledges funding from NHMRC ECF: GNT1143412.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This article is part of the Topical Collection on Pediatric Rheumatology
Rights and permissions
About this article
Cite this article
Harapas, C.R., Steiner, A., Davidson, S. et al. An Update on Autoinflammatory Diseases: Inflammasomopathies. Curr Rheumatol Rep 20, 40 (2018). https://doi.org/10.1007/s11926-018-0750-4
Published:
DOI: https://doi.org/10.1007/s11926-018-0750-4