Abstract
Purpose of Review
To provide a detailed overview of cardiovascular adverse events associated with the use of tyrosine kinase inhibitors across different tumor types.
Recent Findings
Despite an undeniable survival advantage of tyrosine kinase inhibitors (TKIs) in patients with hematologic or solid malignancies, the accompanying off-target cardiovascular adverse events can be life-threatening. In patients with B cell malignancies, the use of Bruton tyrosine kinase inhibitors has been associated with atrial and ventricular arrhythmias, as well as hypertension. Cardiovascular toxic profiles are heterogeneous among the several approved breakpoint cluster region (BCR)-ABL TKIS. Notably, imatinib might be cardioprotective. Vascular endothelial growth factor TKIs, constituting the central axis in the treatment of several solid tumors, including renal cell carcinoma and hepatocellular carcinoma, have strongly been associated with hypertension and arterial ischemic events. Epidermal growth factor TKIs as therapy for advanced non-small cell lung cancer (NSCLC) have been reported to be infrequently associated with heart failure and QT prolongation.
Summary
While tyrosine kinase inhibitors have been demonstrated to increase overall survival across different types of cancers, special consideration should be given to cardiovascular toxicities. High-risk patients can be identified by undergoing a comprehensive workup at baseline.



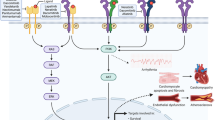
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Du Z, Lovely CM. Mechanisms of receptor tyrosine kinase activation in cancer. Mol Cancer. 2018;17(1):58.
Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–37.
Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenström’s macroglobulinemia. N Engl J Med. 2015;372(15):1430–40.
Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125(16):2497–506.
Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507–16.
Miklos D, Cutler CS, Arora M, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. 2017;130(21):2243–50.
O’Brian S, Jones JA, Coutre S, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17(10):1409–18.
Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395(10232):1278–91.
McMullen JR, Boey EJ, Ooi JY, et al. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood. 2014;124(25):3829–30.
Abbas HA, Wierda W. Acalabrutinib: a selective bruton tyrosine kinase inhibitor for the treatment of B-cell malignancies. Front Oncol. 2021;11: 668162.
Jiang L, Li L, Ruan Y, et al. Ibrutinib promotes atrial fibrillation by inducing structural remodeling and calcium dysregulation in the atrium. Heart Rhythm. 2019;16(9):1374–82.
• Xiao L, Salem JE, Clauss S, et al. Ibrutinib-mediated atrial fibrillation attributable to inhibition of C-terminal Src kinase. Circulation. 2020;142(25):2443–55. Findings from this study suggest that inhibition of the C-terminal Src kinase is an underlying mechanism for atrial fibrillation induced by imatinib.
Pineda-Gayoso R, Alomar M, Lee DH, et al. Cardiovascular toxicities of Bruton’s tyrosine kinase inhibitors. Curr Treat Options Oncol. 2020;21(8):67.
Brown JR, Hillmen P, O’Brien S, et al. Extended follow-up and impact of high-risk prognostic factors from the phase 3 RESONATE study in patients with previously treated CLL/SLL. Leukemia. 2018;32(1):83–91.
Burger JA, Barr PM, Robak T, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;34(3):787–98.
Wiczer TE, Levine LB, Brumbaugh J, et al. Cumulative incidence, risk factors, and management of atrial fibrillation in patients receiving ibrutinib. Blood Adv. 2017;1(20):1739–48.
Batiste F, Cautela J, Ancedy Y, et al. High incidence of atrial fibrillation in patients treated with ibrutinib. Open Heart. 2019;6(1):e001049.
Byrd JC, Hillmen P, Ghia P, et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J Clin Oncol. 2021;39(31):3441–52.
Brown JR, Moslehi J, O’Brien S, et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017;102(10):1796–805.
Reda G, Fattizzo B, Cassin R, et al. Predictors of atrial fibrillation in ibrutinib-treated CLL patients: a prospective study. J Hematol Oncol. 2018;11(1):79.
Mato AR, Clasen S, Pickens P, et al. Left atrial abnormality (LAA) as a predictor of ibrutinib-associated atrial fibrillation in patients with chronic lymphocytic leukemia. Cancer Biol Ther. 2018;19(1):1–2.
Fradley MG, Gliksman M, Emole J, et al. Rates and risk of atrial arrhythmias in patients treated with ibrutinib compared with cytotoxic chemotherapy. Am J Cardiol. 2019;124(4):539–44.
D’Souza M, Carlson N, Fosbøl E, et al. CHA 2 DS 2-VASc score and risk of thromboembolism and bleeding in patients with atrial fibrillation and recent cancer. Eur J Prev Cardiol. 2018;25(6):651–8.
Lipsky A, Lamanna N. Hematology Am Soc Hematol Educ Program. 2020;2020(1):336–45.
Wang ML, Blum KA, Martin P, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood. 2015;126(6):739–45.
Guha A, Derbala MH, Zhao Q, et al. Ventricular arrhythmias following ibrutinib initiation for lymphoid malignancies. J Am Coll Cardiol. 2018;72(6):697–8.
Salem JE, Manouchehri A, Bretagne M, et al. Cardiovascular toxicities associated with ibrutinib. J Am Coll Cardiol. 2019;74(13):1667–78.
O’Brien S, Hillmen P, Coutre S, et al. Safety analysis of four randomized controlled studies of ibrutinib in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma or mantle cell lymphoma. Clin Lymphoma Myeloma Leuk. 2018;18(10):648–57.
Dickerson T, Wiczer T, Waller A, et al. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood. 2019;134(22):1919–28.
Chen ST, Azali L, Rosen L, et al. Hypertension and incident cardiovascular events after next-generation BTKi therapy initiation. J Hematol Oncol. 2022;15(1):92.
• Abdel-Qadir H, Sabrie N, Leong D, et al. Cardiovascular risk associated with ibrutinib use in chronic lymphocytic leukemia: a population-based cohort study. J Clin Oncol. 2021;39(31):3453–62. Findings from this real-world study suggest that ibrutinib is associated with high risks of atrial fibrillation, bleeding, and heart failure but not stroke or acute myocardial infarction.
Giles FJ, Mauro MJ, Hong F, et al. Rates of peripheral arterial occlusive disease in patients with chronic myeloid leukemia in the chronic phase treated with imatinib, nilotinib, or non-tyrosine kinase therapy: a retrospective cohort analysis. Leukemia. 2013;27(6):1310–5.
Shah AM, Campbell P, Querejeta Rocha G, et al. Effect of imatinib as add-on therapy on echocardiographic measures of right ventricular function in patients with significant pulmonary arterial hypertension. Eur Heart J. 2015;36(10):623–32.
Gustafson D, Fish JE, Lipton JH, et al. Mechanisms of cardiovascular toxicity of BCR-ABL1 tyrosine kinase inhibitors in chronic myelogenous leukemia. Curr Hematol Malig Rep. 2020;15(1):20–30.
Trent JC, Patel SS, Zhang J, et al. Rare incidence of congestive heart failure in gastrointestinal stromal tumor and other sarcoma patients receiving imatinib mesylate. Cancer. 2010;116(1):184–92.
Chintalgattu V, Patel SS, Khakoo AY, et al. Cardiovascular effects of tyrosine kinase inhibitors used for gastrointestinal stromal tumors. Hematol Oncol Clin North Am. 2009;23(1):97–107.
National Comprehensive Cancer Network: Chronic myeloid leukemia (Version 3.2022). 2022. https://www.nccn.org/professionals/physician_gls/pdf/cml.pdf.
Guignabert C, Phan C, Seferian A, et al. Dasatinib induces lung vascular toxicity and predisposes to pulmonary hypertension. J Clin Invest. 2016;126(9):3207–18.
Montani D, Bergot E, Günther S, et al. Pulmonary arterial hypertension in patients treated by dasatinib. Circulation. 2012;125(17):2128–37.
Weatherald J, Chaumais M, Savale L, et al. Long-term outcomes of dasatinib-induced pulmonary arterial hypertension: a population-based study. Eur Respir J. 2017;50(1):1700217.
Baumgart B, Guha M, Hennan J, et al. In vitro and in vivo evaluation of dasatinib and imatinib on physiological parameters of pulmonary arterial hypertension. Cancer Chemother Pharmacol. 2017;79(4):711–23.
Shah NP, Kantarjian HM, Kim DW, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol. 2008;26(19):3204–12.
•• Kantarjian HM, Hughes TP, Larson RA, et al. Long-term outcomes with frontline nilotinib versus imatinib in newly diagnosed chronic myeloid leukemia in chronic phase: ENESTnd 10-year analysis. Leukemia. 2021;35(2):440–53. Findings from this study suggest that nilotinib is associated with higher rates of ischemic heart disease, cerebrovascular events, and peripheral arterial disease than imatinib.
Alhawiti N, Burbury KL, Kwa FA, et al. The tyrosine kinase inhibitor, nilotinib potentiates a prothrombotic state. Thromb Res. 2016;145:54–64.
Sadiq S, Owen E, Foster T, et al. Nilotinib-induced metabolic dysfunction: insights from a translational study using in vitro adipocyte models and patient cohorts. Leukemia. 2019;33(7):1810–4.
Kota V, Brümmendorf TH, Gambacorti-Passerini C, et al. Efficacy and safety following bosutinib dose reduction in patients with Philadelphia chromosome-positive leukemias. Leuk Res. 2021;111: 106690.
Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369(19):1783–96.
Cortes JE, Kim DW, Pinilla-Ibarz J, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132(4):393–404.
Cortes J, Apperley J, Lomaia E, et al. Ponatinib dose-ranging study in chronic-phase chronic myeloid leukemia: a randomized, open-label phase 2 clinical trial. Blood. 2021;138(21):2042–50.
Scemblix United States Prescribing Information. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215358s000Orig1lbl.pdf.
Réa D, Mauro M, Boquimpani C, et al. A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after 2 or more prior TKIs. Blood. 2021;138(21):2031–41.
Briasoulis A, Chasouraki A, Sianis A, et al. Cardiotoxicity of non-anthracycline cancer chemotherapy agents. J Cardiovasc Dev Dis. 2022;9(3):66.
Guha A, Sayegh N, Agarwal N. Targeting cardiovascular adverse events of metastatic renal cell carcinoma therapies. JACC CardioOncol. 2022;4(2):235–7.
Dobbin SJH, Petrie MC, Myles RC, et al. Cardiotoxic effects of angiogenesis inhibitors. Clin Sci (Lond). 2021;135(1):71–100.
Hamnvik OR, Choueiri TK, Turchin A, et al. Clinical risk factors for the development of hypertension in patients treated with inhibitors of the VEGF signaling pathway. Cancer. 2015;121(2):311–9.
Zhu X, Stergiopoulos K, Wu S, et al. Risk of hypertension and renal dysfunction with an angiogenesis inhibitor sunitinib: systematic review and meta-analysis. Acta Oncol. 2009;48(1):9–17.
Liu B, Ding F, Yang Liu Y, et al. Incidence and risk of hypertension associated with vascular endothelial growth factor receptor tyrosine kinase inhibitors in cancer patients: a comprehensive network meta-analysis of 72 randomized controlled trials involving 30013 patients. Oncotarget. 2016;7(41):67661–73.
Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807):1931–9.
Qi WX, Lin F, Sun YJ, et al. Incidence and risk of hypertension with pazopanib in patients with cancer: a meta-analysis. Cancer Chemother Pharmacol. 2013;71(2):431–9.
Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res. 2001;49(3):568–81.
González-Pacheco FR, Deudero JJP, Castellanos MC, et al. Mechanisms of endothelial response to oxidative aggression: protective role of autologous VEGF and induction of VEGFR2 by H2O2. Am J Physiol Heart Circ Physiol. 2006;291(3):H1395–401.
Choueiri TK, Schutz F, Je Y, et al. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J Clin Oncol. 2010;28(13):2280–5.
Sonpavde G, Ye J, Schutz F, et al. Venous thromboembolic events with vascular endothelial growth factor receptor tyrosine kinase inhibitors: a systematic review and meta-analysis of randomized clinical trials. Crit Rev Oncol Hematol. 2013;87(1):80–9.
Tran H, Anand SS. Oral antiplatelet therapy in cerebrovascular disease, coronary artery disease, and peripheral arterial disease. JAMA. 2004;292(15):1867–74.
Totzeck M, Mincu RI, Mrotzek S, et al. Cardiovascular diseases in patients receiving small molecules with anti-vascular endothelial growth factor activity: a meta-analysis of approximately 29,000 cancer patients. Eur J Prev Cardiol. 2018;25(5):482–94.
Qi WX, Shen Z, Tang LN, Yao Y. Congestive heart failure risk in cancer patients treated with vascular endothelial growth factor tyrosine kinase inhibitors: a systematic review and meta-analysis of 36 clinical trials. Br J Clin Pharmacol. 2014;78(4):748–62.
Zang J, Wu S, Tang L, et al. Incidence and risk of QTc interval prolongation among cancer patients treated with vandetanib: a systematic review and meta-analysis. PLoS ONE. 2012;7(2): e30353.
Shah RR, Morganroth J, Shah DR. Cardiovascular safety of tyrosine kinase inhibitors: with a special focus on cardiac repolarisation (QT interval). Drug Saf. 2013;36(5):295–316.
Sagie A, Larson MG, Goldberg RJ, et al. An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study). Am J Cardiol. 1992;70(7):797–801.
Chitturi KR, Burns EA, Muhsen IN, et al. Cardiovascular risks with epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors and monoclonal antibody therapy. Curr Oncol Rep. 2022;24(4):475–91.
Thomas A, Rajan A, Giaccone G. Tyrosine kinase inhibitors in lung cancer. Hematol Oncol Clin North Am. 2012;26(3):589–605.
Metibemu DS, Akinloye OA, Akamo AJ, et al. Exploring receptor tyrosine kinases-inhibitors in cancer treatments. Egypt J Med Hum Genet. 2019;20(1):35.
Fukuoka M, Yano S, Giaccone G, et al. J Clin Oncol Official J Am Soc Clin Oncol. 2019;21(12):2237–46.
Orphanos GS, Ioannidis GN, Ardavanis AG. Cardiotoxicity induced by tyrosine kinase inhibitors. Acta Oncol. 2009;48(7):964–70.
Yamaguchi K, Kanazawa S, Kinoshita Y, et al. Acute myocardial infarction with lung cancer during treatment with gefitinib: the possibility of gefitinib-induced thrombosis. Pathophysiol Haemost Thromb. 2003;34(1):48–50.
Lynch DR, Kickler TS, Rade JJ. Recurrent myocardial infarction associated with gefitinib therapy. J Thromb Thrombolysis. 2011;32(1):120–4.
Omori S, Oyakawa T, Naito T, Takahashi T. Gefitinib-induced cardiomyopathy in epidermal growth receptor-mutated NSCLC. J Thorac Oncol. 2018;13(10):e207–8.
Truell JS, Fishbein MC, Figlin R. Myocarditis temporally related to the use of gefitinib (Iressa). Arch Pathol Lab Med. 2005;129(8):1044–6.
Korashy HM, Attafi IM, Ansari MA, et al. Molecular mechanisms of cardiotoxicity of gefitinib in vivo and in vitro rat cardiomyocyte: role of apoptosis and oxidative stress. Toxicol Lett. 2016;252:50–61.
Gronich N, Lavi I, Barnett-Griness O, et al. Tyrosine kinase-targeting drugs-associated heart failure. Br J Cancer. 2017;116(10):1366–73.
Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–6.
Walker AJ, Card TR, West J, et al. Incidence of venous thromboembolism in patients with cancer - a cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49(6):1404–13.
Zaborowska-Szmit M, Krzakowski M, Kowalski DM, Szmit S. Cardiovascular complications of systemic therapy in non-small-cell lung cancer. J Clin Med. 2020;9(5):1268.
Pinquié F, Chabot G, Urban T, Hureaux J. Maintenance treatment by erlotinib and toxic cardiomyopathy: a case report. Oncology. 2016;90(3):176–7.
Kus T, Aktas G, Sevinc A, et al. Could erlotinib treatment lead to acute cardiovascular events in patients with lung adenocarcinoma after chemotherapy failure? Onco Targets Ther. 2015;8:1341–3.
Herbst RS, Ansari R, Bustin F, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): A double-blind, placebo-controlled, phase 3 trial. The Lancet. 2011;377(9780):1846–54.
Cicènas S, Geater SL, Petrov P, et al. Maintenance erlotinib versus erlotinib at disease progression in patients with advanced non-small-cell lung cancer who have not progressed following platinum-based chemotherapy (IUNO study). Lung Cancer. 2016;102:30–7.
Ewer MS, Patel K, O’Brien D, Lorence RM. Cardiac safety of afatinib: a review of data from clinical trials. Cardio-Oncology. 2015;1(1):3.
Nuvola G, Dall’Olio FG, Melotti B, et al. Cardiac toxicity from afatinib in EGFR-mutated NSCLC: a rare but possible side effect. J Thorac Oncol. 2019;14(7):e145–e146.
Tang Z, Ji X, Zhou G, et al. Hypotension from afatinib in epidermal growth factor receptor-mutated non-small cell lung cancer: a case report and literature review. Anticancer Drugs. 2022;33(1):e840–1.
Cross DAE, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4(9):1046–61.
Kunimasa K, Kamada R, Oka T, et al. Cardiac adverse events in EGFR-mutated non-small cell lung cancer treated with osimertinib. JACC Cardio-Oncology. 2020;2(1):1–10.
Thein KZ, Swarup S, Ball S, et al. Incidence of cardiac toxicities in patients with advanced non-small cell lung cancer treated with osimertinib: a combined analysis of two phase III randomized controlled trials. Ann Oncol. 2018;29(supp_8):VIII500.
Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113–25.
Tagrisso tablets 40 mg, Tagrisso tablets 80 mg, the result of the use-results surveys final report. 2019 [in Japanese]. https://www.mhlw.go.jp/content/11120000/000484135.pdf.
Mok TS, Wu Y-L, Ahn M-J, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–40.
Anand K, Ensor J, Trachtenberg B, Bernicker EH. Osimertinib-induced cardiotoxicity: a retrospective review of the FDA adverse events reporting system (FAERS). JACC Cardio-Oncology. 2019;1(2):172–8.
Kunimasa K, Oka T, Hara S, et al. Osimertinib is associated with reversible and dose-independent cancer therapy-related cardiac dysfunction. Lung Cancer (Amsterdam, Netherlands). 2021;153:186–92.
Lyon AR, Dent S, Stanway S, et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur J Heart Fail. 2020;22(11):1945–60.
Alexandre J, Cautela J, Ederhy S, et al. Cardiovascular toxicity related to cancer treatment: a pragmatic approach to the American and European cardio-oncology guidelines. J Am Heart Assoc. 2020;9(18): e018403.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Neeraj Agarwal reports personal fees from Astellas, Astra Zeneca, Aveo, Bayer, Bristol Myers Squibb, Calithera, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Foundation Medicine, Genentech, Gilead, Janssen, Merck, MEI Pharma, Nektar, Novartis, Pfizer, Pharmacyclics, Seattle Genetic. They also report grants from Astellas, Astra Zeneca, Bavarian Nordic, Bayer, Bristol Myers Squibb, Calithera, Celldex, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Genentech, Gilead, Glaxo Smith Kline, Immunomedics, Janssen, Medivation, Merck, Nektar, New Link Genetics, Novartis, Pfizer, Prometheus, Rexahn, Roche, Sanofi, Seattle Genetics, Takeda, and Tracon, outside the submitted work. Daniel Addison reports grants from American Heart Association, NHLBI-NIH, and Pelotonia, outside the submitted work. Jorge Cortes reports grants and personal fees from BMS, Novartis, Pfizer, Takeda, Daiichi, Jazz Pharmaceuticals, Merus, and Forma Therapeutics; grants from Astellas and Amphivena; and personal fees from BiolineRx and Bioptah, outside the submitted work. Neal L Weintraub reports grants from NIH-NHLBI and the American Heart Association, outside the submitted work. Nazish Sayed reports grants from NIH-NHLBI and the American Heart Association, outside the submitted work. Avirup Guha reports personal fees from Pfizer/Myovant and grants from the American Heart Association, outside the submitted work. The other authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardio-Oncology
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sayegh, N., Yirerong, J., Agarwal, N. et al. Cardiovascular Toxicities Associated with Tyrosine Kinase Inhibitors. Curr Cardiol Rep 25, 269–280 (2023). https://doi.org/10.1007/s11886-023-01845-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-023-01845-2