Abstract
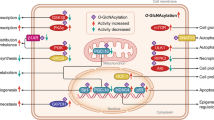
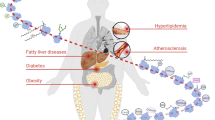
The protein prenylation is one of the essential post-translational protein modifications, which extensively exists in the eukaryocyte. It includes protein farnesylation and geranylgeranylation, using farnesyl pyrophosphate (FPP) or geranylgeranyl pyrophosphate (GGPP) as the substrate, respectively. The prenylation occurs by covalent addition of these two types of isoprenoids to cysteine residues at or near the carboxyl terminus of the proteins that possess CaaX motif, such as Ras small GTPase family. The attachment of hydrophobic prenyl groups can anchor the proteins to intracellular membranes and trigger downstream cell signaling pathway. Geranylgeranyl biphosphate synthase (GGPPS) catalyzes the synthesis of 20-carbon GGPP from 15-carbon FPP. The abnormal expression of this enzyme will affect the relative content of FPP and GGPP, and thus disrupts the balance between protein farnesylation and geranylgeranylation, which participates into various aspects of cellular physiology and pathology. In this paper, we mainly review the property of this important protein post-translational modification and research progress in its regulation of cigarette smoke induced pulmonary disease, adipocyte insulin sensitivity, the inflammation response of Sertoli cells, the hepatic lipogenesis and the cardiac hypertrophy.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
McTaggart S. Isoprenylated proteins. Cell Mol Life Sci, 2006, 63: 255–267
Perez-Sala D. Protein isoprenylation in biology and disease: general overview and perspectives from studies with genetically engineered animals. Front Biosci, 2007, 12: 4456–4472
Rodríguez-Concepción M, Boronat A. Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids. A metabolic milestone achieved through genomics. Plant Physiol, 2002, 130: 1079–1089
Eisenreich W, Bacher A, Arigoni D, Rohdich F. Biosynthesis of isoprenoids via the non-mevalonate pathway. Cell Mol Life Sci, 2004, 61: 1401–1426
Vranová E, Coman D, Gruissem W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Ann Rev Plant Biol, 2013, 64: 665–700
Maurer-Stroh S, Washietl S, Eisenhaber F. Protein prenyltransferases. Genome Biol, 2003, 4: 212
Berndt N, Hamilton AD, Sebti SM. Targeting protein prenylation for cancer therapy. Nat Rev Cancer, 2011, 11: 775–791
Carboni JM, Yan N, Cox AD, Bustelo X, Graham SM, Lynch MJ, Weinmann R, Seizinger BR, Der CJ, Barbacid M. Farnesyltransferase inhibitors are inhibitors of ras but not r-ras2/tc21, transformation. Oncogene, 1995, 10: 1905–1913
Whyte DB, Kirschmeier P, Hockenberry TN, Nunez-Oliva I, James L, Catino JJ, Bishop WR, Pai J-K. K-and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J Biol Chem, 1997, 272: 14459–14464
Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer, 2003, 3: 11–22
Mijimolle N, Velasco J, Dubus P, Guerra C, Weinbaum CA, Casey PJ, Campuzano V, Barbacid M. Protein farnesyltransferase in embryogenesis, adult homeostasis, and tumor development. Cancer Cell, 2005, 7: 313–324
Sjogren AK, Andersson KM, Liu M, Cutts BA, Karlsson C, Wahlstrom AM, Dalin M, Weinbaum C, Casey PJ, Tarkowski A, Swolin B, Young SG, Bergo MO. GGTase-I deficiency reduces tumor formation and improves survival in mice with K-Ras-induced lung cancer. J Clin Invest, 2007, 117: 1294–1304
Khan OM, Ibrahim MX, Jonsson IM, Karlsson C, Liu M, Sjogren AK, Olofsson FJ, Brisslert M, Andersson S, Ohlsson C, Hulten LM, Bokarewa M, Bergo MO. Geranylgeranyltransferase type I (GGTase-I) deficiency hyperactivates macrophages and induces erosive arthritis in mice. J Clin Invest, 2011, 121: 628–639
Shotelersuk V, Gahl WA. Hermansky-pudlak syndrome: models for intracellular vesicle formation. Mol Genet Metab, 1998, 65: 85–96
Larijani B, Hume AN, Tarafder AK, Seabra MC. Multiple factors contribute to inefficient prenylation of Rab27a in Rab prenylation diseases. J Biol Chem, 2003, 278: 46798–46804
Pereira-Leal JB, Hume AN, Seabra MC. Prenylation of rab GTPases: molecular mechanisms and involvement in genetic disease. FEBS Lett, 2001, 498: 197–200
Weivoda MM, Hohl RJ. Geranylgeranyl pyrophosphate stimulates PPARγ expression and adipogenesis through the inhibition of osteoblast differentiation. Bone, 2012, 50: 467–476
Weivoda MM, Hohl RJ. The effects of direct inhibition of geranylgeranyl pyrophosphate synthase on osteoblast differentiation. J Cell Biochem, 2011, 112: 1506–1513
Li CJ, Ning W, Matthay MA, Feghali-Bostwick CA, Choi AM. MAPK pathway mediates EGR-1-HSP70-dependent cigarette smoke-induced chemokine production. Am J Physiol-Lung Cell Mol Physiol, 2007, 292: L1297–1303
Ning W, Dong Y, Sun J, Li C, Matthay MA, Feghali-Bostwick CA, Choi AM. Cigarette smoke stimulates matrix metalloproteinase-2 activity via EGR-1 in human lung fibroblasts. Am J Resp Cell Mol Biol, 2007, 36: 480
Ning W, Li CJ, Kaminski N, Feghali-Bostwick CA, Alber SM, Di YP, Otterbein SL, Song R, Hayashi S, Zhou Z, Pinsky DJ, Watkins SC, Pilewski JM, Sciurba FC, Peters DG, Hogg JC, Choi AM. Comprehensive gene expression profiles reveal pathways related to the pathogenesis of chronic obstructive pulmonary disease. Proc Natl Acad Sci USA, 2004, 101: 14895–14900
Shen N, Shao Y, Lai SS, Qiao L, Yang RL, Xue B, Pan FY, Chen HQ, Li CJ. GGPPS, a new EGR-1 target gene, reactivates ERK 1/2 signaling through increasing ras prenylation. Am J Pathol, 2011, 179: 2740–2750
Chen H, Wang L, Gong T, Yu Y, Zhu C, Li F, Wang L, Li C. EGR-1 regulates Ho-1 expression induced by cigarette smoke. Biochem Biophys Res Commun, 2010, 396: 388–393
Shen N, Gong T, Wang JD, Meng FL, Qiao L, Yang RL, Xue B, Pan FY, Zhou XJ, Chen HQ, Ning W, Li CJ. Cigarette smoke-induced pulmonary inflammatory responses are mediated by EGR-1/GGPPS/MAPK signaling. Am J Pathol, 2011, 178: 110–118
Hotamisligil GS. Inflammation and metabolic disorders. Nature, 2006, 444: 860–867
Vicent D, Maratos-Flier E, Kahn CR. The branch point enzyme of the mevalonate pathway for protein prenylation is overexpressed in the ob/ob mouse and induced by adipogenesis. Mol Cell Biol, 2000, 20: 2158–2166
Solomon C, Leitner J, Goalstone M. Dominant negative α-subunit of farnesyl-and geranylgeranyl-transferase I inhibits insulin-induced differentiation of 3T3-L1 pre-adipocytes. Int J Obes Relat Metab Disord, 2003, 27: 40–47
Shen N, Yu X, Pan FY, Gao X, Xue B, Li CJ. An early response transcription factor, EGR-1, enhances insulin resistance in type 2 diabetes with chronic hyperinsulinism. J Biol Chem, 2011, 286: 14508–14515
Yu X, Shen N, Zhang ML, Pan FY, Wang C, Jia WP, Liu C, Gao Q, Gao X, Xue B, Li CJ. EGR-1 decreases adipocyte insulin sensitivity by tilting PI3K/Akt and MAPK signal balance in mice. EMBO J, 2011, 30: 3754–3765
Brennan J, Capel B. One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat Rev Genet, 2004, 5: 509–521
Mackay S. Gonadal development in mammals at the cellular and molecular levels. Int Rev Cytol, 2000, 200: 47–99
Philip J, Selvan D, Desmond AD. Mumps orchitis in the non-immune postpubertal male: a resurgent threat to male fertility? BJU Int, 2006, 97: 138–141
Setchell BP. Blood-testis barrier, junctional and transport proteins and spermatogenesis. Adv Exp Med Biol, 2008, 636: 212–233
Petersen C, Söder O. The sertoli cell—a hormonal target and ‘super’nurse for germ cells that determines testicular size. Horm Res Paediatr, 2006, 66: 153–161
Sun B, Qi N, Shang T, Wu H, Deng T, Han D. Sertoli cell-initiated testicular innate immune response through toll-like receptor-3 activation is negatively regulated by Tyro3, Axl, and mer receptors. Endocrinology, 2010, 151: 2886–2897
Riccioli A, Starace D, Galli R, Fuso A, Scarpa S, Palombi F, De Cesaris P, Ziparo E, Filippini A. Sertoli cells initiate testicular innate immune responses through TLR activation. J Immunol, 2006, 177: 7122–7130
Wang XX, Ying P, Diao F, Wang Q, Ye D, Jiang C, Shen N, Xu N, Chen WB, Lai SS, Jiang S, Miao XL, Feng J, Tao WW, Zhao NW, Yao B, Xu ZP, Sun HX, Li JM, Sha JH, Huang XX, Shi QH, Tang H, Gao X, Li CJ. Altered protein prenylation in sertoli cells is associated with adult infertility resulting from childhood mumps infection. J Exp Med, 2013, 210: 1559–1574
Frith J, Day CP, Robinson L, Elliott C, Jones DE, Newton JL. Potential strategies to improve uptake of exercise interventions in non-alcoholic fatty liver disease. J Hepatol, 2010, 52: 112–116
Wang L, Shen ZH, Xu Q. Study on the prevalence of nonalcoholic fatty liver and the association between nonalcoholic fatty liver and metabolic syndrome. Chin J Convalesc Med, 2010, 5: 067
Kotronen A, Westerbacka J, Bergholm R, Pietiläinen KH, Yki-Järvinen H. Liver fat in the metabolic syndrome. J Clin Endocrinol Metab, 2007, 92: 3490–3497
Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology, 2003, 37: 1202–1219
Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, Moore DD, Auwerx J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest, 2004, 113: 1408–1418
Hunter J, Tanaka N, Rockman H, Ross J, Chien K. Ventricular expression of a MLC-2v-ras fusion gene induces cardiac hypertrophy and selective diastolic dysfunction in transgenic mice. J Biol Chem, 1995, 270: 23173
Sah VP, Minamisawa S, Tam SP, Wu TH, Dorn GW, Ross J, Chien KR, Brown JH. Cardiac-specific overexpression of RhoA results in sinus and atrioventricular nodal dysfunction and contractile failure. J Clin Invest, 1999, 103: 1627–1634
Heineke J, Molkentin J. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol, 2006, 7: 589–600
Porter KE, Turner NA. Statins and myocardial remodelling: cell and molecular pathways. Expert Rev Mol Med, 2011, 13: e22
Bergo MO, Lieu HD, Gavino BJ, Ambroziak P, Otto JC, Casey PJ, Walker QM, Young SG. On the physiological importance of endoproteolysis of CAAX proteins. J Biol Chem, 2004, 279: 4729
Yang J, Mou Y, Wu T, Ye Y, Jiang JC, Zhao CZ, Zhu HH, Du CQ, Zhou L, Hu SJ. Cardiac-specific overexpression of farnesyl pyrophosphate synthase induces cardiac hypertrophy and dysfunction in mice. Cardiovasc Res, 2013, 97: 490–499
Li L, Chen G, Yang Y, Ye Y, Yao L, Hu S. Chronic inhibition of farnesyl pyrophosphate synthase attenuates cardiac hypertrophy and fibrosis in spontaneously hypertensive rats. Biochem Pharmacol, 2010, 79: 399–406
Yi P, Han Z, Li X, Olson E. The mevalonate pathway controls heart formation in Drosophila by isoprenylation of Ggamma 1. Science, 2006, 313: 1301
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics C, Stroke Statistics S. Executive summary: heart disease and stroke statistics—2013 update: a report from the american heart association. Circulation, 2013, 127: 143–152
Xu N, Guan S, Chen Z, Yu Y, Xie J, Pan FY, Zhao NW, Liu L, Yang ZZ, Gao X, Xu B, Li CJ. The alteration of protein prenylation induces cardiomyocyte hypertrophy through Rheb-mTORC1 signalling and leads to chronic heart failure. J Pathol, 2014, doi: 10.1002/path.4480
Lane KT, Beese LS. Thematic review series: lipid posttranslational modifications. Structural biology of protein farnesyltransferase and geranylgeranyltransferase type I. J Lipid Res, 2006, 47: 681–699
Edwards PA, Kast HR, Anisfeld AM. Bareing it all: the adoption of LXR and FXR and their roles in lipid homeostasis. J Lipid Res, 2002, 43: 2–12
Kalaany NY, Mangelsdorf DJ. LXRS and FXR: The yin and yang of cholesterol and fat metabolism. Ann Rev Physiol, 2006, 68: 159–191
Id Boufker H, Lagneaux L, Fayyad-Kazan H, Badran B, Najar M, Wiedig M, Ghanem G, Laurent G, Body JJ, Journé F. Role of farnesoid X receptor (FXR) in the process of differentiation of bone marrow stromal cells into osteoblasts. Bone, 2011, 49: 1219–1231
Bełtowski J. Liver X receptors (LXR) as therapeutic targets in dyslipidemia. Cardiovasc Ther, 2008, 26: 297–316
Mitro N, Mak PA, Vargas L, Godio C, Hampton E, Molteni V, Kreusch A, Saez E. The nuclear receptor LXR is a glucose sensor. Nature, 2006, 445: 219–223
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published with open access at link.springer.com
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Xu, N., Shen, N., Wang, X. et al. Protein prenylation and human diseases: a balance of protein farnesylation and geranylgeranylation. Sci. China Life Sci. 58, 328–335 (2015). https://doi.org/10.1007/s11427-015-4836-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-015-4836-1