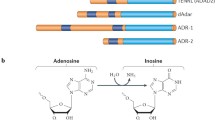
Abstract
Adenosine to inosine (A-to-I) RNA editing is the most abundant editing event in animals. It converts adenosine to inosine in double-stranded RNA regions through the action of the adenosine deaminase acting on RNA (ADAR) proteins. Editing of pre-mRNA coding regions can alter the protein codon and increase functional diversity. However, most of the A-to-I editing sites occur in the non-coding regions of pre-mRNA or mRNA and non-coding RNAs. Untranslated regions (UTRs) and introns are located in pre-mRNA non-coding regions, thus A-to-I editing can influence gene expression by nuclear retention, degradation, alternative splicing, and translation regulation. Non-coding RNAs such as microRNA (miRNA), small interfering RNA (siRNA) and long non-coding RNA (lncRNA) are related to pre-mRNA splicing, translation, and gene regulation. A-to-I editing could therefore affect the stability, biogenesis, and target recognition of non-coding RNAs. Finally, it may influence the function of non-coding RNAs, resulting in regulation of gene expression. This review focuses on the function of ADAR-mediated RNA editing on mRNA non-coding regions (UTRs and introns) and non-coding RNAs (miRNA, siRNA, and lncRNA).
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Lee J T. Epigenetic regulation by long noncoding RNAs. Science, 2012, 338: 1435–1439
Noller H F. Ribosomal RNA and translation. Annu Rev Biochem, 1991, 60: 191–227
Dahlberg A E. The functional role of ribosomal RNA in protein synthesis. Cell, 1989, 57: 525–529
Rich A, RajBhandary U L. Transfer RNA: Molecular structure, sequence, and properties. Annu Rev Biochem, 1976, 45: 805–860
Bachellerie J P, Cavaille J, Huttenhofer A. The expanding snoRNA world. Biochimie, 2002, 84: 775–790
Fabian M R, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem, 2010, 79: 351–379
Kishore S, Stamm S. Regulation of alternative splicing by snoRNAs. Cold Spring Harb Symp Quant Biol, 2006, 71: 329–334
McKeown M. The role of small nuclear RNAs in RNA splicing. Curr Opin Cell Biol, 1993, 5: 448–454
Kishore S, Stamm S. The snoRNA HBII-52 regulates alternative splicing of the serotonin receptor 2C. Science, 2006, 311: 230–232
Hirota K, Miyoshi T, Kugou K, et al. Stepwise chromatin remodelling by a cascade of transcription initiation of non-coding RNAs. Nature, 2008, 456: 130–134
Eulalio A, Huntzinger E, Izaurralde E. Getting to the root of miRNA-mediated gene silencing. Cell, 2008, 132: 9–14
Liu Q, Paroo Z. Biochemical principles of small RNA pathways. Annu Rev Biochem, 2010, 79: 295–319
Hermeking H. microRNAs in the p53 network: Micromanagement of tumour suppression. Nat Rev Cancer, 2012, 12: 613–626
Croce C M, Calin G A. miRNAs, cancer, and stem cell division. Cell, 2005, 122: 6–7
Lujambio A, Lowe S W. The microcosmos of cancer. Nature, 2012, 482: 347–355
Sahoo T, del Gaudio D, German J R, et al. Prader-Willi phenotype caused by paternal deficiency for the HBII-85 C/D box small nucleolar RNA cluster. Nat Genet, 2008, 40: 719–721
Ding F, Li H H, Zhang S, et al. SnoRNA Snord116 (Pwcr1/MBII-85) deletion causes growth deficiency and hyperphagia in mice. PLoS ONE, 2008, 3: e1709
Nakatani J, Tamada K, Hatanaka F, et al. Abnormal behavior in a chromosome-engineered mouse model for human 15q11-13 duplication seen in autism. Cell, 2009, 137: 1235–1246
Bazeley P S, Shepelev V, Talebizadeh Z, et al. snoTARGET shows that human orphan snoRNA targets locate close to alternative splice junctions. Gene, 2008, 408: 172–179
Faghihi M A, Modarresi F, Khalil A M, et al. Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of beta-secretase. Nat Med, 2008, 14: 723–730
Gott J M, Emeson R B. Functions and mechanisms of RNA editing. Annu Rev Genet, 2000, 34: 499–531
Nishikura K. Functions and regulation of RNA editing by ADAR deaminases. Annu Rev Biochem, 2010, 79: 321–349
Peng Z, Cheng Y, Tan B C, et al. Comprehensive analysis of RNA-Seq data reveals extensive RNA editing in a human transcriptome. Nat Biotechnol, 2012, 30: 253–260
Pullirsch D, Jantsch M F. Proteome diversification by adenosine to inosine RNA editing. RNA Biol, 2010, 7: 205–212
Bass B L. RNA editing by adenosine deaminases that act on RNA. Annu Rev Biochem, 2002, 71: 817–846
Bass B L, Weintraub H. An unwinding activity that covalently modifies its double-stranded RNA substrate. Cell, 1988, 55: 1089–1098
Wagner R W, Smith J E, Cooperman B S, et al. A double-stranded RNA unwinding activity introduces structural alterations by means of adenosine to inosine conversions in mammalian cells and Xenopus eggs. Proc Natl Acad Sci USA, 1989, 86: 2647–2651
Paul M S, Bass B L. Inosine exists in mRNA at tissue-specific levels and is most abundant in brain mRNA. EMBO J, 1998, 17: 1120–1127
Chen C X, Cho D S, Wang Q, et al. A third member of the RNA-specific adenosine deaminase gene family, ADAR3, contains both single- and double-stranded RNA binding domains. RNA, 2000, 6: 755–767
Hough R F, Lingam A T, Bass B L. Caenorhabditis elegans mRNAs that encode a protein similar to ADARs derive from an operon containing six genes. Nucleic Acids Res, 1999, 27: 3424–3432
Palladino M J, Keegan L P, O’Connell M A, et al. dADAR, a Drosophila double-stranded RNA-specific adenosine deaminase is highly developmentally regulated and is itself a target for RNA editing. RNA, 2000, 6: 1004–1018
Gerber A, Grosjean H, Melcher T, et al. Tad1p, a yeast tRNA-specific adenosine deaminase, is related to the mammalian pre-mRNA editing enzymes ADAR1 and ADAR2. EMBO J, 1998, 17: 4780–4789
Auxilien S, Crain P F, Trewyn R W, et al. Mechanism, specificity and general properties of the yeast enzyme catalysing the formation of inosine 34 in the anticodon of transfer RNA. J Mol Biol, 1996, 262: 437–458
Maas S, Gerber A P, Rich A. Identification and characterization of a human tRNA-specific adenosine deaminase related to the ADAR family of pre-mRNA editing enzymes. Proc Natl Acad Sci USA, 1999, 96: 8895–8900
Maas S, Kim Y G, Rich A. Sequence, genomic organization and functional expression of the murine tRNA-specific adenosine deaminase ADAT1. Gene, 2000, 243: 59–66
Keegan L P, Gerber A P, Brindle J, et al. The properties of a tRNA-specific adenosine deaminase from Drosophila melanogaster support an evolutionary link between pre-mRNA editing and tRNA modification. Mol Cell Biol, 2000, 20: 825–833
Jin Y, Zhang W, Li Q. Origins and evolution of ADAR-mediated RNA editing. IUBMB Life, 2009, 61: 572–578
Tonkin L A, Saccomanno L, Morse D P, et al. RNA editing by ADARs is important for normal behavior in Caenorhabditis elegans. EMBO J, 2002, 21: 6025–6035
Palladino M J, Keegan L P, O’Connell M A, et al. A-to-I pre-mRNA editing in Drosophila is primarily involved in adult nervous system function and integrity. Cell, 2000, 102: 437–449
Palavicini J P, O’Connell M A, Rosenthal J J. An extra double-stranded RNA binding domain confers high activity to a squid RNA editing enzyme. RNA, 2009, 15: 1208–1218
Slavov D, Clark M, Gardiner K. Comparative analysis of the RED1 and RED2 A-to-I RNA editing genes from mammals, pufferfish and zebrafish. Gene, 2000, 250: 41–51
Slavov D, Crnogorac-Jurcevic T, Clark M, et al. Comparative analysis of the DRADA A-to-I RNA editing gene from mammals, pufferfish and zebrafish. Gene, 2000, 250: 53–60
Herbert A, Alfken J, Kim Y G, et al. A Z-DNA binding domain present in the human editing enzyme, double-stranded RNA adenosine deaminase. Proc Natl Acad Sci USA, 1997, 94: 8421–8426
Lehmann K A, Bass B L. The importance of internal loops within RNA substrates of ADAR1. J Mol Biol, 1999, 291: 1–13
Nishikura K, Yoo C, Kim U, et al. Substrate specificity of the dsRNA unwinding/modifying activity. EMBO J, 1991, 10: 3523–3532
Wong S K, Sato S, Lazinski D W. Substrate recognition by ADAR1 and ADAR2. RNA, 2001, 7: 846–858
Tian N, Yang Y, Sachsenmaier N, et al. A structural determinant required for RNA editing. Nucleic Acids Res, 2011, 39: 5669–5681
Enstero M, Daniel C, Wahlstedt H, et al. Recognition and coupling of A-to-I edited sites are determined by the tertiary structure of the RNA. Nucleic Acids Res, 2009, 37: 6916–6926
Stefl R, Oberstrass F C, Hood J L, et al. The solution structure of the ADAR2 dsRBM-RNA complex reveals a sequence-specific readout of the minor groove. Cell, 2010, 143: 225–237
Melcher T, Maas S, Herb A, et al. RED2, a brain-specific member of the RNA-specific adenosine deaminase family. J Biol Chem, 1996, 271: 31795–31798
Kim U, Wang Y, Sanford T, et al. Molecular cloning of cDNA for double-stranded RNA adenosine deaminase, a candidate enzyme for nuclear RNA editing. Proc Natl Acad Sci USA, 1994, 91: 11457–11461
O’Connell M A, Krause S, Higuchi M, et al. Cloning of cDNAs encoding mammalian double-stranded RNA-specific adenosine deaminase. Mol Cell Biol, 1995, 15: 1389–1397
Gerber A, O’Connell M A, Keller W. Two forms of human double-stranded RNA-specific editase 1 (hRED1) generated by the insertion of an Alu cassette. RNA, 1997, 3: 453–463
Lai F, Chen C X, Carter K C, et al. Editing of glutamate receptor B subunit ion channel RNAs by four alternatively spliced DRADA2 double-stranded RNA adenosine deaminases. Mol Cell Biol, 1997, 17: 2413–2424
Melcher T, Maas S, Herb A, et al. A mammalian RNA editing enzyme. Nature, 1996, 379: 460–464
Patterson J B, Samuel C E. Expression and regulation by interferon of a double-stranded-RNA-specific adenosine deaminase from human cells: Evidence for two forms of the deaminase. Mol Cell Biol, 1995, 15: 5376–5388
Desterro J M, Keegan L P, Lafarga M, et al. Dynamic association of RNA-editing enzymes with the nucleolus. J Cell Sci, 2003, 116: 1805–1818
Poulsen H, Nilsson J, Damgaard C K, et al. CRM1 mediates the export of ADAR1 through a nuclear export signal within the Z-DNA binding domain. Mol Cell Biol, 2001, 21: 7862–7871
Sansam C L, Wells K S, Emeson R B. Modulation of RNA editing by functional nucleolar sequestration of ADAR2. Proc Natl Acad Sci USA, 2003, 100: 14018–14023
Juhling F, Morl M, Hartmann R K, et al. tRNAdb 2009: Compilation of tRNA sequences and tRNA genes. Nucleic Acids Res, 2009, 37: D159–162
Gerber A P, Keller W. An adenosine deaminase that generates inosine at the wobble position of tRNAs. Science, 1999, 286: 1146–1149
Wolf J, Gerber A P, Keller W. tadA, an essential tRNA-specific adenosine deaminase from Escherichia coli. EMBO J, 2002, 21: 3841–3851
Keller W, Wolf J, Gerber A. Editing of messenger RNA precursors and of tRNAs by adenosine to inosine conversion. FEBS Lett, 1999, 452: 71–76
Danecek P, Nellaker C, McIntyre R E, et al. High levels of RNA-editing site conservation amongst 15 laboratory mouse strains. Genome Biol, 2012, 13: 26
Zhang Z, Carmichael G G. The fate of dsRNA in the nucleus: a p54(nrb)-containing complex mediates the nuclear retention of promiscuously A-to-I edited RNAs. Cell, 2001, 106: 465–475
DeCerbo J, Carmichael G G. Retention and repression: Fates of hyperedited RNAs in the nucleus. Curr Opin Cell Biol, 2005, 17: 302–308
Prasanth K V, Prasanth S G, Xuan Z, et al. Regulating gene expression through RNA nuclear retention. Cell, 2005, 123: 249–263
Chen L L, DeCerbo J N, Carmichael G G. Alu element-mediated gene silencing. EMBO J, 2008, 27: 1694–1705
Chen L L, Carmichael G G. Gene regulation by SINES and inosines: Biological consequences of A-to-I editing of Alu element inverted repeats. Cell Cycle, 2008, 7: 3294–3301
Hundley H A, Krauchuk A A, Bass B L. C. elegans and H. sapiens mRNAs with edited 3′ UTRs are present on polysomes. RNA, 2008, 14: 2050–2060
Capshew C R, Dusenbury K L, Hundley H A. Inverted Alu dsRNA structures do not affect localization but can alter translation efficiency of human mRNAs independent of RNA editing. Nucleic Acids Res, 2012, 40: 8637–8645
Chen L L, Carmichael G G. Altered nuclear retention of mRNAs containing inverted repeats in human embryonic stem cells: Functional role of a nuclear noncoding RNA. Mol Cell, 2009, 35: 467–478
Roberts L, Holcik M. RNA structure: New messages in translation, replication and disease. Workshop on the role of RNA structures in the translation of viral and cellular RNAs. EMBO Rep, 2009, 10: 449–453
Levanon E Y, Eisenberg E, Yelin R, et al. Systematic identification of abundant A-to-I editing sites in the human transcriptome. Nat Biotechnol, 2004, 22: 1001–1005
Athanasiadis A, Rich A, Maas S. Widespread A-to-I RNA editing of Alu-containing mRNAs in the human transcriptome. PLoS Biol, 2004, 2: e391
Bartel D P. microRNAs: Target recognition and regulatory functions. Cell, 2009, 136: 215–233
Liang H, Landweber L F. Hypothesis: RNA editing of microRNA target sites in humans? RNA, 2007, 13: 463–467
Scadden A D. The RISC subunit Tudor-SN binds to hyper-edited double-stranded RNA and promotes its cleavage. Nat Struct Mol Biol, 2005, 12: 489–496
Scadden A D, Smith C W. Specific cleavage of hyper-edited dsRNAs. EMBO J, 2001, 20: 4243–4252
Yang W, Chendrimada T P, Wang Q, et al. Modulation of microRNA processing and expression through RNA editing by ADAR deaminases. Nat Struct Mol Biol, 2006, 13: 13–21
Weissbach R, Scadden A D. Tudor-SN and ADAR1 are components of cytoplasmic stress granules. RNA, 2012, 18: 462–471
Scadden A D. Inosine-containing dsRNA binds a stress-granule-like complex and downregulates gene expression in trans. Mol Cell, 2007, 28: 491–500
Wang Q, Miyakoda M, Yang W, et al. Stress-induced apoptosis associated with null mutation of ADAR1 RNA editing deaminase gene. J Biol Chem, 2004, 279: 4952–4961
Hartner J C, Walkley C R, Lu J, et al. ADAR1 is essential for the maintenance of hematopoiesis and suppression of interferon signaling. Nat Immunol, 2009, 10: 109–115
Toth A M, Li Z, Cattaneo R, et al. RNA-specific adenosine deaminase ADAR1 suppresses measles virus-induced apoptosis and activation of protein kinase PKR. J Biol Chem, 2009, 284: 29350–29356
Rueter S M, Dawson T R, Emeson R B. Regulation of alternative splicing by RNA editing. Nature, 1999, 399: 75–80
Valente L, Nishikura K. ADAR gene family and A-to-I RNA editing: diverse roles in posttranscriptional gene regulation. Prog Nucleic Acid Res Mol Biol, 2005, 79: 299–338
Lev-Maor G, Sorek R, Levanon E Y, et al. RNA-editing-mediated exon evolution. Genome Biol, 2007, 8: R29
Moller-Krull M, Zemann A, Roos C, et al. Beyond DNA: RNA editing and steps toward Alu exonization in primates. J Mol Biol, 2008, 382: 601–609
Lander E S, Linton L M, Birren B, et al. Initial sequencing and analysis of the human genome. Nature, 2001, 409: 860–921
Batzer M A, Deininger P L. Alu repeats and human genomic diversity. Nat Rev Genet, 2002, 3: 370–379
Greenberger S, Levanon E Y, Paz-Yaacov N, et al. Consistent levels of A-to-I RNA editing across individuals in coding sequences and non-conserved Alu repeats. BMC Genomics, 2010, 11: 608
Kim D D, Kim T T, Walsh T, et al. Widespread RNA editing of embedded alu elements in the human transcriptome. Genome Res, 2004, 14: 1719–1725
Blow M, Futreal P A, Wooster R, et al. A survey of RNA editing in human brain. Genome Res, 2004, 14: 2379–2387
Fukui T, Itoh M. RNA editing in P transposable element read-through transcripts in Drosophila melanogaster. Genetica, 2010, 138: 1119–1126
Peters N T, Rohrbach J A, Zalewski B A, et al. RNA editing and regulation of Drosophila 4f-rnp expression by sas-10 antisense readthrough mRNA transcripts. RNA, 2003, 9: 698–710
Fischer S E, Butler M D, Pan Q, et al. Trans-splicing in C. elegans generates the negative RNAi regulator ERI-6/7. Nature, 2008, 455: 491–496
Limbach P A, Crain P F, McCloskey J A. Summary: The modified nucleosides of RNA. Nucleic Acids Res, 1994, 22: 2183–2196
Carthew R W, Sontheimer E J. Origins and mechanisms of miRNAs and siRNAs. Cell, 2009, 136: 642–655
Luciano D J, Mirsky H, Vendetti N J, et al. RNA editing of a miRNA precursor. RNA, 2004, 10: 1174–1177
Kawahara Y, Zinshteyn B, Chendrimada T P, et al. RNA editing of the microRNA-151 precursor blocks cleavage by the Dicer-TRBP complex. EMBO Rep, 2007, 8: 763–769
Kawahara Y, Megraw M, Kreider E, et al. Frequency and fate of microRNA editing in human brain. Nucleic Acids Res, 2008, 36: 5270–5280
Heale B S, Keegan L P, McGurk L, et al. Editing independent effects of ADARs on the miRNA/siRNA pathways. EMBO J, 2009, 28: 3145–3156
Ota H, Sakurai M, Gupta R, et al. ADAR1 forms a complex with Dicer to promote microRNA processing and RNA-induced gene silencing. Cell, 2013, 153: 575–589
Iizasa H, Wulff B E, Alla N R, et al. Editing of Epstein-Barr virus-encoded BART6 microRNAs controls their dicer targeting and consequently affects viral latency. J Biol Chem, 2010, 285: 33358–33370
Kawahara Y, Zinshteyn B, Sethupathy P, et al. Redirection of silencing targets by adenosine-to-inosine editing of miRNAs. Science, 2007, 315: 1137–1140
Scadden A D, Smith C W. RNAi is antagonized by A→I hyper-editing. EMBO Rep, 2001, 2: 1107–1111
Tonkin L A, Bass B L. Mutations in RNAi rescue aberrant chemotaxis of ADAR mutants. Science, 2003, 302: 1725
Knight S W, Bass B L. The role of RNA editing by ADARs in RNAi. Mol Cell, 2002, 10: 809–817
Yang W, Wang Q, Howell K L, et al. ADAR1 RNA deaminase limits short interfering RNA efficacy in mammalian cells. J Biol Chem, 2005, 280: 3946–3953
Silhavy D, Molnar A, Lucioli A et al. A viral protein suppresses RNA silencing and binds silencing-generated, 21- to 25-nucleotide double-stranded RNAs. EMBO J, 2002, 21: 3070–3080
Vargason J M, Szittya G, Burgyan J, et al. Size selective recognition of siRNA by an RNA silencing suppressor. Cell, 2003, 115: 799–811
Ye K, Malinina L, Patel D J. Recognition of small interfering RNA by a viral suppressor of RNA silencing. Nature, 2003, 426: 874–878
Li H, Li W X, Ding S W. Induction and suppression of RNA silencing by an animal virus. Science, 2002, 296: 1319–1321
Lu R, Maduro M, Li F, et al. Animal virus replication and RNAi-mediated antiviral silencing in Caenorhabditis elegans. Nature, 2005, 436: 1040–1043
Wilkins C, Dishongh R, Moore S C, et al. RNA interference is an antiviral defence mechanism in Caenorhabditis elegans. Nature, 2005, 436: 1044–1047
Schott D H, Cureton D K, Whelan S P, et al. An antiviral role for the RNA interference machinery in Caenorhabditis elegans. Proc Natl Acad Sci USA, 2005, 102: 18420–18424
Hong J, Qian Z, Shen S, et al. High doses of siRNAs induce eri-1 and adar-1 gene expression and reduce the efficiency of RNA interference in the mouse. Biochem J, 2005, 390: 675–679
Kapranov P, Cheng J, Dike S, et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science, 2007, 316: 1484–1488
Kung J T, Colognori D, Lee J T. Long noncoding RNAs: Past, present, and future. Genetics, 2013, 193: 651–669
Zhao J, Ohsumi T K, Kung J T, et al. Genome-wide identification of polycomb-associated RNAs by RIP-seq. Mol Cell, 2010, 40: 939–953
Novikova I V, Hennelly S P, Sanbonmatsu K Y. Sizing up long non-coding RNAs: Do lncRNAs have secondary and tertiary structure? Bioarchitecture, 2012, 2: 189–199
Gong C, Maquat L E. lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3′ UTRs via Alu elements. Nature, 2011, 470: 284–288
Cesana M, Cacchiarelli D, Legnini I, et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell, 2011, 147: 358–369
Bertani S, Sauer S, Bolotin E, et al. The noncoding RNA Mistral activates Hoxa6 and Hoxa7 expression and stem cell differentiation by recruiting MLL1 to chromatin. Mol Cell, 2011, 43: 1040–1046
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published with open access at Springerlink.com
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Yang, Y., Zhou, X. & Jin, Y. ADAR-mediated RNA editing in non-coding RNA sequences. Sci. China Life Sci. 56, 944–952 (2013). https://doi.org/10.1007/s11427-013-4546-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-013-4546-5