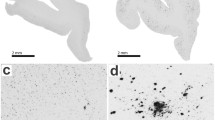
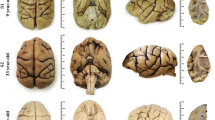
Abstract
At later ages, humans have high risk of developing Alzheimer disease (AD) which may afflict up to 50% by 90 years. While prosimians and monkeys show more substantial changes, the great apes brains examined show mild neurodegenerative changes. Compared with rodents, primates develop and reproduce slowly and are long lived. The New World primates contain some of the shortest as well as some of the longest-lived monkey species, while the prosimians develop the most rapidly and are the shortest lived. Great apes have the largest brains, slowest development, and longest lives among the primates. All primates share some level of slowly progressive, age-related neurodegenerative changes. However, no species besides humans has yet shown regular drastic neuron loss or cognitive decline approaching clinical grade AD. Several primates accumulate extensive deposits of diffuse amyloid-beta protein (Aβ) but only a prosimian—the gray mouse lemur—regularly develops a tauopathy approaching the neurofibrillary tangles of AD. Compared with monkeys, nonhuman great apes display even milder brain-aging changes, a deeply puzzling observation. The genetic basis for these major species differences in brain aging remains obscure but does not involve the Aβ coding sequence which is identical in nonhuman primates and humans. While chimpanzees merit more study, we note the value of smaller, shorter-lived species such as marmosets and small lemurs for aging studies. A continuing concern for all aging studies employing primates is that relative to laboratory rodents, primate husbandry is in a relatively primitive state, and better husbandry to control infections and obesity is needed for brain aging research.





Similar content being viewed by others
References
Abbott DH, Barnett DK, Colman RJ, Yamamoto ME, Schultz-Darken NJ (2003) Aspects of common marmoset basic biology and life history important for biomedical research. Comp Med 53:339–350
Allman J, Rosin A, Kumar R, Hasenstaub A (1998) Parenting and survival in anthropoid primates: caretakers live longer. Proc Natl Acad Sci U S A 95:6866–6869
Altmann J, Gesquiere L, Galbany J, Onyango PO, Alberts SC (2010) Life history context of reproductive aging in a wild primate model. Ann N Y Acad Sci 1204:127–138
Amano T, Meyer JS, Okabe T, Shaw T, Mortel KF (1982) Stable xenon CT cerebral blood flow measurements computed by a single compartment–double integration model in normal aging and dementia. J Comput Assist Tomogr 6:923–932
Anderson US, Stoinski TS, Bloomsmith MA, Maple TL (2007) Relative numerousness judgment and summation in young, middle-aged, and older adult orangutans (Pongo pygmaeus abelii and Pongo pygmaeus pygmaeus). J Comp Psychol 121:1–11
Anderson US, Stoinski TS, Bloomsmith MA, Marr MJ, Smith AD, Maple TL (2005) Relative numerousness judgment and summation in young and old Western lowland gorillas. J Comp Psychol 119:285–295
Arriagada PV, Marzloff K, Hyman BT (1992) Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology 42:1681–1688
Atsalis S, Videan E (2009) Reproductive aging in captive and wild common chimpanzees: factors influencing the rate of follicular depletion. Am J Primatol 71:271–282
Austad SN (2001) Concepts and theories of aging. In: Masoro EJ, Austad SN (eds) Handbook of the biology of aging, 5th edn. Academic, San Diego, pp 3–22
Austad SN, Fischer KE (1991) Mammalian aging, metabolism, and ecology: evidence from the bats and marsupials. J Gerontol 46:B47–B53
Austad SN, Fischer KE (1992) Primate longevity: its place in the mammalian scheme. Am J Primatol 28:251–261
Austad SN, Fischer KE (2011) The development of small primate models for aging research. ILAR J 52:78–88
Beltran WA, Vanore M, Ollivet F, Nemoz-Bertholet F, Aujard F, Clerc B et al (2007) Ocular findings in two colonies of gray mouse lemurs (Microcebus murinus). Vet Ophthalmol 10:43–49
Bininda-Emonds OR, Cardillo M, Jones KE, MacPhee RD, Beck RM, Grenyer R et al (2007) The delayed rise of present-day mammals. Nature 446:507–512
Blalock EM, Grondin R, Chen KC, Thibault O, Thibault V, Pandya JD et al (2010) Aging-related gene expression in hippocampus proper compared with dentate gyrus is selectively associated with metabolic syndrome variables in rhesus monkeys. J Neurosci 30:6058–6071
Bodkin NL, Alexander TM, Ortmeyer HK, Johnson E, Hansen BC (2003) Mortality and morbidity in laboratory-maintained rhesus monkeys and effects of long-term dietary restriction. J Gerontol A Biol Sci Med Sci 58:212–219
Bogin B (1999) Evolutionary perspective on human growth. Ann Rev Anthropol 28:109–156
Bons N, Rieger F, Prudhomme D, Fisher A, Krause KH (2006) Microcebus murinus: a useful primate model for human cerebral aging and Alzheimer’s disease? Genes Brain Behav 5:120–130
Boyd-Kimball D, Sultana R, Mohmmad-Abdul H, Butterfield DA (2004) Rodent Abeta(1-42) exhibits oxidative stress properties similar to those of human Abeta(1-42): implications for proposed mechanisms of toxicity. J Alzheimers Dis 6:515–525
Braak H, Del TK (2011a) Alzheimer’s pathogenesis: is there neuron-to-neuron propagation? Acta Neuropathol 121:589–595
Braak H, Del TK (2011b) The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol 121:171–181
Bronikowski AM, Alberts SC, Altmann J, Packer C, Carey KD, Tatar M (2002) The aging baboon: comparative demography in a non-human primate. Proc Natl Acad Sci U S A 99:9591–9595
Bronikowski AM, Altmann J, Brockman DK, Cords M, Fedigan LM, Pusey A et al (2011) Aging in the natural world: comparative data reveal similar mortality patterns across primates. Science 331:1325–1328
Buffenstein R (2005) The naked mole-rat: a new long-living model for human aging research. J Gerontol A Biol Sci Med Sci 60:1369–1377
Caspari R, Lee SH (2004) Older age becomes common late in human evolution. Proc Natl Acad Sci U S A 101:10895–10900
Chatterjee HJ, Ho SY, Barnes I, Groves C (2009) Estimating the phylogeny and divergence times of primates using a supermatrix approach. BMC Evol Biol 9:259
Chimpanzee Sequencing & Analysis Consortium (2005) Initial sequence of the chimpanzee genome and comparison with the human genome. Nature 437:69–87
Cole GM, Ma QL, Frautschy SA (2010) Dietary fatty acids and the aging brain. Nutr Rev 68(Suppl 2):S102–S111
Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM et al (2009) Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325:201–204
Coulson EJ, Paliga K, Beyreuther K, Masters CL (2000) What the evolution of the amyloid protein precursor supergene family tells us about its function. Neurochem Int 36:175–184
Dhenain M, Michot JL, Privat N, Picq JL, Boller F, Duyckaerts C et al (2000) MRI description of cerebral atrophy in mouse lemur primates. Neurobiol Aging 21:81–88
Dickerson BC, Stoub TR, Shah RC, Sperling RA, Killiany RJ, Albert MS et al (2011) Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology 76:1395–1402
Duan H, Wearne SL, Rocher AB, Macedo A, Morrison JH, Hof PR (2003) Age-related dendritic and spine changes in corticocortically projecting neurons in macaque monkeys. Cereb Cortex 13:950–961
Eisenberg JF (1981) The mammalian radiations. University of Chicago Press, Chicago
Fabre PH, Rodrigues A, Douzery EJ (2009) Patterns of macroevolution among primates inferred from a supermatrix of mitochondrial and nuclear DNA. Mol Phylogenet Evol 53:808–825
Fiala JC, Feinberg M, Peters A, Barbas H (2007) Mitochondrial degeneration in dystrophic neurites of senile plaques may lead to extracellular deposition of fine filaments. Brain Struct Funct 212:195–207
Finch CE (1972) Enzyme activities, gene function and ageing in mammals. (Review). Exp Gerontol 7:53–67
Finch CE (2007) The biology of human longevity: inflammation, nutrition, and aging in the evolution of lifespans. Academic, San Diego
Finch CE (2009) The neurobiology of middle-age has arrived. Neurobiol Aging 30:515–520
Finch CE (2010) Evolution in health and medicine Sackler colloquium: evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition. Proc Natl Acad Sci U S A 107(Suppl 1):1718–1724
Finch CE, Holmes DJ (2010) Ovarian aging in developmental and evolutionary contexts. Ann N Y Acad Sci 1204:82–94
Finch CE, Morgan TE, Rozovsky I, Xie Z, Weindruch R, Prolla T (2002) Microglia and aging in the brain. In: Streit WJ (ed) Microglia in the regenerating and degenerating central nervous system. Springer, New York, pp 275–305
Finch CE, Stanford CB (2004) Meat-adaptive genes and the evolution of slower aging in humans. Q Rev Biol 79:3–50
Fjell AM, Westlye LT, Espeseth T, Reinvang I, Dale AM, Holland D et al (2010) Cortical gray matter atrophy in healthy aging cannot be explained by undetected incipient cognitive disorders: a comment on Burgmans et al. 2009. Neuropsychology 24:258–263
Fukumoto H, Rosene DL, Moss MB, Raju S, Hyman BT, Irizarry MC (2004) Beta-secretase activity increases with aging in human, monkey, and mouse brain. Am J Pathol 164:719–725
Fullerton SM, Clark AG, Weiss KM, Nickerson DA, Taylor SL, Stengard JH et al (2000) Apolipoprotein E variation at the sequence haplotype level: implications for the origin and maintenance of a major human polymorphism. Am J Hum Genet 67:881–900
Gearing M, Rebeck GW, Hyman BT, Tigges J, Mirra SS (1994) Neuropathology and apolipoprotein E profile of aged chimpanzees: implications for Alzheimer disease. Proc Natl Acad Sci USA 91:9382–93826
Gearing M, Tigges J, Mori H, Mirra SS (1996) A beta40 is a major form of beta-amyloid in nonhuman primates. Neurobiol Aging 17:903–908
Gearing M, Tigges J, Mori H, Mirra SS (1997) A beta-amyloid (A beta) deposition in the brains of aged orangutans. Neurobiol Aging 18:139–146
Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER et al (2007) Evolutionary and biomedical insights from the rhesus macaque genome. Science 316:222–234
Gimelbrant A, Hutchinson JN, Thompson BR, Chess A (2007) Widespread monoallelic expression on human autosomes. Science 318:1136–1140
Glazko GV, Nei M (2003) Estimation of divergence times for major lineages of primate species. Mol Biol Evol 20:424–434
Grzimek B (1990) Grzimek’s Encyclopedia of Mammals, vol 2, Parker SP (ed), McGraw-Hill, New York
Haley GE, Kohama SG, Urbanski HF, Raber J (2010) Age-related decreases in SYN levels associated with increases in MAP-2, apoE, and GFAP levels in the rhesus macaque prefrontal cortex and hippocampus. Age (Dordr) 32:283–296
Hansen LA, Armstrong DM, Terry RD (1987) An immunohistochemical quantification of fibrous astrocytes in the aging human cerebral cortex. Neurobiol Aging 8:1–6
Harada N, Nishiyama S, Satoh K, Fukumoto D, Kakiuchi T, Tsukada H (2002) Age-related changes in the striatal dopaminergic system in the living brain: a multiparametric PET study in conscious monkeys. Synapse 45:38–45
Hardy J (2009) The amyloid hypothesis for Alzheimer’s disease: a critical reappraisal. J Neurochem 110:1129–1134
Hawkes K, Paine RR (2006) The evolution of human life history. School of American Research Press, Santa Fe
Hawkes K, Smith KR (2010) Do women stop early? Similarities in fertility decline in humans and chimpanzees. Ann N Y Acad Sci 1204:43–53
Herndon JG, Moss MB, Rosene DL, Killiany RJ (1997) Patterns of cognitive decline in aged rhesus monkeys. Behav Brain Res 87:25–34
Herndon JG, Tigges J, Klumpp SA, Anderson DC (1998) Brain weight does not decrease with age in adult rhesus monkeys. Neurobiol Aging 19:267–272
Hoffman CL, Higham JP, Mas-Rivera A, Ayala JE, Maestripieri D (2010) Terminal investment and senescence in rhesus macaques (Macaca mulatta) on Cayo Santiago. Behav Ecol 21:972–978
Holzer M, Craxton M, Jakes R, Arendt T, Goedert M (2004) Tau gene (MAPT) sequence variation among primates. Gene 341:313–322
Hotta K, Gustafson TA, Ortmeyer HK, Bodkin NL, Nicolson MA, Hansen BC (1996) Regulation of obese (ob) mRNA and plasma leptin levels in rhesus monkeys. Effects of insulin, body weight, and non-insulin-dependent diabetes mellitus. J Biol Chem 271:25327–25331
Ingram DK, Chefer S, Matochik J, Moscrip TD, Weed J, Roth GS et al (2001) Aging and caloric restriction in nonhuman primates: behavioral and in vivo brain imaging studies. Ann N Y Acad Sci 928:316–326
Jacobsen KT, Iverfeldt K (2009) Amyloid precursor protein and its homologues: a family of proteolysis-dependent receptors. Cell Mol Life Sci 66:2299–2318
Ji ZS, Mullendorff K, Cheng IH, Miranda RD, Huang Y, Mahley RW (2006) Reactivity of apolipoprotein E4 and amyloid beta peptide: lysosomal stability and neurodegeneration. J Biol Chem 281:2683–2692
Johnstone EM, Chaney MO, Norris FH, Pascual R, Little SP (1991) Conservation of the sequence of the Alzheimer’s disease amyloid peptide in dog, polar bear and five other mammals by cross-species polymerase chain reaction analysis. Brain Res Mol Brain Res 10:299–305
Judge DS, Carey JR (2000) Postreproductive life predicted by primate patterns. J Gerontol A Biol Sci Med Sci 55:B201–B209
Kadish I, Thibault O, Blalock EM, Chen KC, Gant JC, Porter NM et al (2009) Hippocampal and cognitive aging across the lifespan: a bioenergetic shift precedes and increased cholesterol trafficking parallels memory impairment. J Neurosci 29:1805–1816
Kaplan H, Hill K, Lancaster JA, Hurtado AM (2000) A theory of human life history: diet, intelligence, and longevity. Evolutionary Anthropology 9:156–185
Kimura N, Nakamura S, Goto N, Narushima E, Hara I, Shichiri S, Saitou K, Nose M, Hayashi T, Kawamura S, Yoshikawa Y (2001) Senile plaques in an aged western lowland gorilla. Exp Anim 50:77–81
Kirkness EF, Bafna V, Halpern AL, Levy S, Remington K, Rusch DB et al (2003) The dog genome: survey sequencing and comparative analysis. Science 301:1898–1903
Klein WL, Krafft GA, Finch CE (2001) Targeting small Abeta oligomers: the solution to an Alzheimer’s disease conundrum? Trends Neurosci 24:219–224
Klimentidis YC, Beasley TM, Lin HY, Murati G, Glass GE, Guyton M, Newton W, Jorgensen M, Heymsfield SB, Kemnitz J, Fairbanks L, Allison DB (2011) Canaries in the coal mine: a cross-species analysis of the plurality of obesity epidemics. Proc Biol Sci 278:1626–1632
Kohler IV, Preston SH, Lackey LB (2006) Comparative mortality levels among selected species of captive animals. Demogr Res 15:413–434
Kraska A, Dorieux O, Picq JL, Petit F, Bourrin E, Chenu E, Volk A, Perret M, Hantraye P, Mestre-Frances N, Aujard F, Dhenain M (2011) Age-associated cerebral atrophy in mouse lemur primates. Neurobiol Aging 32:894–906
Lacreuse A, Kim CB, Rosene DL, Killiany RJ, Moss MB, Moore TL et al (2005) Sex, age, and training modulate spatial memory in the rhesus monkey (Macaca mulatta). Behav Neurosci 119:118–126
Leduc V, Jasmin-Belanger S, Poirier J (2010) APOE and cholesterol homeostasis in Alzheimer’s disease. Trends Mol Med 16:469–477
Li KZ, Lindenberger U, Freund AM, Baltes PB (2001) Walking while memorizing: age-related differences in compensatory behavior. Psychol Sci 12:230–237
Luebke J, Barbas H, Peters A (2010) Effects of normal aging on prefrontal area 46 in the rhesus monkey. Brain Res Rev 62:212–232
Lutermann H, Schmelting B, Radespiel U, Ehresmann P, Zimmermann E (2006) The role of survival for the evolution of female philopatry in a solitary forager, the grey mouse lemur (Microcebus murinus). Proc R Soc B 273:2527–2533
Mahley RW, Weisgraber KH, Huang Y (2009) Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer’s disease to AIDS. J Lipid Res 50(Suppl):S183–S188
Maloney B, Ge YW, Greig N, Lahiri DK (2004) Presence of a “CAGA box” in the APP gene unique to amyloid plaque-forming species and absent in all APLP-1/2 genes: implications in Alzheimer’s disease. FASEB J 18:1288–1290
Mansfield K (2003) Marmoset models commonly used in biomedical research. Comp Med 53:383–392
Marchal S, Givalois L, Verdier JM, Mestre-Francés N (2012) Distribution of lithostathine in the mouse lemur brain with aging and Alzeheimer’s-like pathology. Neurobiol Aging 33:431.e15–25
Martin LJ, Mahaney MC, Bronikowski AM, Carey KD, Dyke B, Comuzzie AG (2002) Lifespan in captive baboons is heritable. Mech Age Devel 123:1461–1467
Masliah E, Mallory M, Hansen L, DeTeresa R, Terry RD (1993) Quantitative synaptic alterations in the human neocortex during normal aging. Neurology 43:192–197
McArdle JJ (2009) Latent variable modeling of differences and changes with longitudinal data. Annu Rev Psychol 60:577–605
McDonald CR, McEvoy LK, Gharapetian L, Fennema-Notestine C, Hagler DJ Jr, Holland D et al (2009) Regional rates of neocortical atrophy from normal aging to early Alzheimer disease. Neurology 73:457–465
Moerman ML (1982) Growth of the birth canal in adolescent girls. Am J Obstet Gynecol 143:528–532
Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB (2006) Executive system dysfunction occurs as early as middle-age in the rhesus monkey. Neurobiol Aging 27:1484–1493
Morgan DG, Marcusson JO, Nyberg P, Wester P, Winblad B, Gordon MN et al (1987) Divergent changes in D-1 and D-2 dopamine binding sites in human brain during aging. Neurobiol Aging 8:195–201
Murphy WJ, Pevzner PA, O’Brien SJ (2004) Mammalian phylogenomics comes of age. Trends Genet 20:631–639
Nowak RM (1999) Walker’s mammals of the world, 6th edn. Johns Hopkins University Press, Baltimore
Ohm TG, Muller H, Braak H, Bohl J (1995) Close-meshed prevalence rates of different stages as a tool to uncover the rate of Alzheimer’s disease-related neurofibrillary changes. Neuroscience 64:209–217
Picq JL (2007) Aging affects executive functions and memory in mouse lemur primates. Exp Gerontol 42:223–232
Picq JL, Aujard F, Volk A, Dhenain M (2011) Age-related cerebral atrophy in nonhuman primates predicts cognitive impairments. Neurobiol Aging (in press)
Prasher VP, Farrer MJ, Kessling AM, Fisher EM, West RJ, Barber PC et al (1998) Molecular mapping of Alzheimer-type dementia in Down’s syndrome. Ann Neurol 43:380–383
Raffai RL, Dong LM, Farese RV Jr, Weisgraber KH (2001) Introduction of human apolipoprotein E4 “domain interaction” into mouse apolipoprotein E. Proc Natl Acad Sci U S A 98:11587–11591
Rapp PR, Amaral DG (1991) Recognition memory deficits in a subpopulation of aged monkeys resemble the effects of medial temporal lobe damage. Neurobiol Aging 12:481–486
Remick AK, Van Wettere AJ, Williams CV (2009) Neoplasia in prosimians: case series from a captive prosimian population and literature review. Vet Pathol 46:746–772
Robbins MM, Bermejo M, Cipolletta C, Magliocca F, Parnell RJ, Stokes E (2004) Social structure and life-history patterns in western gorillas (Gorilla gorilla gorilla). Am J Primatol 64:145–159
Robson SL, Wood B (2008) Hominin life history: reconstruction and evolution. J Anat 212:394–425
Rosen RD (2008) The lie of the jungle. Washington Post, 7 December 2008, p. W14
Rosen RF, Farberg AS, Gearing M, Dooyema J, Long PM, Anderson DC et al (2008) Tauopathy with paired helical filaments in an aged chimpanzee. J Comp Neurol 509:259–270
Rovelet-Lecrux A, Hannequin D, Raux G, Le MN, Laquerriere A, Vital A et al (2006) APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet 38:24–26
Rowe N (1996) Pictorial guide to the living primates. Pogonias Press, East Hampton
Rozovsky I, Wei M, Morgan TE, Finch CE (2005) Reversible age impairments in neurite outgrowth by manipulations of astrocytic GFAP. Neurobiol Aging 26:705–715
Salthouse TA (2009) When does age-related cognitive decline begin? Neurobiol Aging 30:507–514
Schultz C, Hubbard GB, Rüb U, Braak E, Braak H (2000) Age-related progression of tau pathology in brains of baboons. Neurobiol Aging 21:905–912
Severson JA, Finch CE (1980) Reduced dopaminergic binding during aging in the rodent striatum. Brain Res 192:147–162
Severson JA, Marcusson J, Winblad B, Finch CE (1982) Age-correlated loss of dopaminergic binding sites in human basal ganglia. J Neurochem 39:1623–1631
Shamy JL, Habeck C, Hof PR, Amaral DG, Fong SG, Buonocore MH et al (2011) Volumetric correlates of spatiotemporal working and recognition memory impairment in aged rhesus monkeys. Cereb Cortex 21:1559–1573
Sherwood CC, Gordon AD, Allen JS, Phillips KE, Erwin JW, Hof PW, et al. (2011) Aging of the cerebral cortex differs between humans and chimpanzees. Proc Natl Acad Sci U S A (in press)
Simmons HA, Mattison JA (2011) The incidence of spontaneous neoplasia in two populations of captive rhesus macaques (Macaca mulatta). Antioxid Redox Signal 14:221–227
Simonian NA, Hyman BT (1994) Functional alterations in Alzheimer’s disease: selective loss of mitochondrial-encoded cytochrome oxidase mRNA in the hippocampal formation. J Neuropathol Exp Neurol 53:508–512
Smucny DA, Abbott DH, Mansfield KG, Schultz-Darken NJ, Yamamoto ME, Alencar AI et al (2004) Reproductive output, maternal age, and survivorship in captive common marmoset females (Callithrix jacchus). Am J Primatol 64:107–121
Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B et al (2010) The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS One 5:e9505
Steinetz BG, Randolph C, Cohn D, Mahoney CJ (1996) Lipoprotein profiles and glucose tolerance in lean and obese chimpanzees. J Med Primatol 25:17–25
Strong R, Miller RA, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, Nadon NL, Warner HR, Harrison DE (2008) Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell 7:641–650
Sullivan PM, Mace BE, Estrada JC, Schmechel DE, Alberts MJ (2008) Human apolipoprotein E4 targeted replacement mice show increased prevalence of intracerebral hemorrhage associated with vascular amyloid deposition. J Stroke Cerebrovasc Dis 17:303–311
Tardif SD, Mansfield KG, Ratnam R, Ross CN, Ziegler TE (2011) The marmoset as a model of aging and age-related diseases. ILAR J 52:54–65
Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW (1999) Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci 54:B492–B501
Vamathevan JJ, Hasan S, Emes RD, Amrine-Madsen H, Rajagopalan D, Topp SD et al (2008) The role of positive selection in determining the molecular cause of species differences in disease. BMC Evol Biol 8:273
Varki N, Anderson D, Herndon JG, Pham T, Gregg CJ, Cheriyan M et al (2009) Heart disease is common in humans and chimpanzees, but is caused by different pathological processes. Evol Appl 2:101–112
Varki NM, Strobert E, Dick EJ Jr, Benirschke K, Varki A (2011) Biomedical differences between human and nonhuman hominids: potential roles for uniquely human aspects of sialic acid biology. Annu Rev Pathol 6:365–393
Vaughan TA, Ryan JM, Czaplewski NJ (2000) Mammalogy, 4th edn. Harcourt, Orlando
Walsh DM, Selkoe DJ (2007) A beta oligomers—a decade of discovery. J Neurochem 101:1172–1184
Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P et al (2002) Initial sequencing and comparative analysis of the mouse genome. Nature 420:520–562
Wich SA, Shumaker RW, Perkins L, de Vries H (2009) Captive and wild orangutan (Pongo sp.) survivorship: a comparison and the influence of management. Am J Primatol 71:680–686
Wich SA, Utami-Atmoko SS, Setia TM, Rijksen HD, Schurmann C, van Hooff JA et al (2004) Life history of wild Sumatran orangutans (Pongo abelii). J Hum Evol 47:385–398
Williams-Blangero S, Butler T, Brasky K, Murthy KK (1994) Heritabilities of clinical chemical traits in chimpanzees. Lab Anim Sci 44:141–143
Yamagiwa J (1997) Mushamuka’s story: the largest group and the longest tenure. Gorilla J 15:7–9
Zanjani H, Finch CE, Kemper C, Atkinson J, McKeel D, Morris JC et al (2005) Complement activation in very early Alzheimer disease. Alzheimer Dis Assoc Disord 19:55–66
Author information
Authors and Affiliations
Corresponding author
Additional information
In, AGE journal special issue “Nonhuman Primate Models of Aging” Ilhem Messaoudi, Ph.D. (Guest Editor) messaoud@ohsu.edu
About this article
Cite this article
Finch, C.E., Austad, S.N. Primate aging in the mammalian scheme: the puzzle of extreme variation in brain aging. AGE 34, 1075–1091 (2012). https://doi.org/10.1007/s11357-011-9355-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-011-9355-9