Abstract
Our study designed to study the potential of potassium dichromate (K2Cr2O7) oral exposure to induce damage in male rat brain and to compare the possible protective role of vitamin C (VC) either pre and/or concurrent supply against (K2Cr2O7) induced changes. Thirty male rats were divided into five groups. First control group received distilled water (C), second received 120 mg/kg b.wt (VC), third received 25 mg/kg b.wt K2Cr2O7 (Cr), fourth group received VC together with K2Cr2O7 by the same former doses (VC + Cr), and the fifth group received the same oral doses of VC 2 weeks prior to and along with K2Cr2O7 for 6 weeks (VC + Cr pro/co treated). The obtained results revealed that K2Cr2O7 induced a significant decrease in cholinergic activity, glutathione reductase GR activity, reduced glutathione content GSH and ATP levels. Furthermore, K2Cr2O7 induced a significant increase in oxidative DNA damage indicated by 8-hydroxy 2′-deoxyguanosine (8OH2′dG) and formation of apoptotic DNA ladders, significant increase in malondialdehyde (MDA), protein carbonyl, and lactate dehydrogenase enzyme. Increased mRNA expression of pro-apoptotic genes, including caspase-3, p53, and Bax, unlike Bcl-2 expression, was decreased. K2Cr2O7 increased caspase-3 and decreased Bcl-2 immuno-labeling. VC supply noticeably ameliorates K2Cr2O7-induced changes which were more significantly in VC pro and concurrent supplement rather than VC concurrent supply only. Finally, it is concluded that K2Cr2O7 oral administration induced oxidative apoptotic changes in rat brain and confirms the usefulness of VC pre and concurrent supply for the amelioration of K2Cr2O7-induced events more significantly than VC concurrent supply only.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Potassium-dichromate (K2Cr2O7) is one of the soluble hexavalent chromium compounds which is widely used and found in effluents of more than 50 different industries worldwide including pigment and textile production, leather tanneries, wood processing, chrome plating, metallurgical and chemical industries, stainless steel factories, welding, cement manufacturing factories, ceramic, glass, and photographic industries, catalytic converter production for automobiles, heat resistance, and as an anti-rust agent in cooling plants (Costa and Klein 2006). Cr (VI) is released into the environment to contaminate the air, rivers, vegetables, fish, drinking water, meat, and milk (Wartelle and Marshall 2005). Cr (VI) enters the environmental air and can be discharged into the water from chromium-containing wastes (Nickens et al. 2010). Furthermore, water contaminated with hexavalent Cr is a worldwide problem, as it is the major route of exposure for the general population (Nudler et al. 2009). Cr (VI) present also in food directly interacting with gastric mucosa DNA (Trzeciak et al. 2000) and modifying the expression of cancer-inducing genes (Tsao et al. 2011).
The nervous system is a commonly vulnerable organ to exposure either to essential or toxic nonessential metals, which readily accumulates metals resulting in noxious consequences such as apoptosis. An extensive literature is available on Cr-induced cytotoxic effects on several organs of different mammals; nevertheless, little data was available about Cr neurological impact neither in humans or animals from exposure to Cr (III) nor in animals from exposure to Cr (VI). And, no histopathological lesions were observed in the brain, spinal cord, or nerve tissue of rats exposed to Cr (VI) concentration of 15.5 mg/m3 for 2 years (Wilbur et al. 2000). No neurological tests were done. Previous evidence showed for the first time that Cr accumulates in rat hypothalamus and anterior pituitary cells after Cr VI oral administration (500 ppm) causing apoptosis mainly due to oxidative stress generation (Quinteros et al. 2008, 2007). Dashti et al. (2016) reported that K2Cr2O7-induced oxidative stress and neurotoxic impact in cultured cerebral granule neurons more significantly in mature than immature neurons.
Vitamin C (VC) is a potent water-soluble antioxidant vitamin that scavenges reactive oxygen species (ROS) and reduces oxidative stress in vitro and in vivo (Tamari et al. 2013). The antioxidant properties of ascorbate are well established as a reducing agent, donating electrons to various enzymatic and a few non-enzymatic reactions. Being an essential co-factor for many enzymes, VC is simply required for maintenance of healthy life (Rekha et al. 2012). Furthermore, being an efficient scavenger of reactive oxygen species (ROS) and free radicals, vitamin C exerts a protective role against many environmental carcinogenic compounds and even some types of cancer (Kucharski and Zajac 2009).
Aim of this work
Taking in consideration these previous data, and because of little-published data that demonstrate brain injury induced by Cr VI oral administration in male rats, thus the present in vivo study is done to assess the comparative potential of vitamin C pro and/or concurrent administration as a simple, natural antioxidant to ameliorate Cr VI-induced apoptosis in rat brain.
Materials and methods
Animals
Laboratory animal needed for this study were 30 adult male Sprague–Dawley rats weighing about (160–200 g). Rats were obtained from the Laboratory Animal’s farm of Faculty of Veterinary Medicine, Zagazig University. Rats were housed in plastic cages with a photoperiod of a 12-h light/12-h dark cycle, a minimum relative humidity of 50%, and a room temperature of 22–28 °C. Food and water were available ad libitum. All the experimental protocol is endorsed by the Approval of Ethics Committee of Faculty of Veterinary Medicine, Zagazig University, in accordance with the guiding principles of the National Institutes of Health (NIH) for the Care and Use of Laboratory Animals.
Chemicals
Potassium dichromate (K2Cr2O7), CAS No: 7778-50-9 (purity, 99%), and vitamin C (Cevarol, ampoules 200 mg/ml) were purchased from El-Nasr pharmaceutical chemicals company ADWICK, Egypt. All other reagents used were of analytical grade and obtained from Sigma (St. Louis, MO, USA).
Animal treatment
Rats were divided into five equal groups (n=6) of each. First control group (C) orally received distilled water, second vitamin C-treated group (VC) orally received vitamin C in dose of 120 mg/kg BW (Qureshi and Mahmood 2010), third Cr-treated group (Cr) orally received K2Cr2O7 at dose level of 25 mg/kg BW according to that used by Krim et al. (2013) for 6 weeks, fourth group exposed to Cr together with VC simultaneously for 6 weeks (Cr + VC co-treated) with the same mentioned doses and route. The fifth group protective and co-treated with VC for 2 weeks prior to and along with Cr exposure for 6 weeks (VC + Cr pro/co-treated) simultaneously by the same previous doses, route, and duration.
Sampling and preparation of brain tissue homogenates
At the end of the experimental period, rats from different groups were anesthetized by inhalation of diethyl ether. The brain tissue specimens were dissected from the skull and rinsed with sterile physiological saline (0.9%, NaCl). Subsequently, half of brains collected from all differnt experimental groups were snap-frozen by immersion in liquid nitrogen and kept at (− 80 °C) until gene expression analysis of apoptosis-related genes (P53, Bax, Bcl-2, and caspase-3).
For evaluation of oxidative stress biomarkers, acetylcholinesterase (AChE; EC 3.1.1.7), lactate dehydrogenase (LDH; EC 1.1.1.27), 8-hydroxy-2-deoxyguanosine and total ATP content, 500 mg of brain tissues collected from all experimental groups were homogenized for 5 min in 0.115 M chilled phosphate buffer saline (1:5 w/v) using tissue homogenizer (Potter–Elvehjem), and then, the homogenates were centrifuged at 14,000 rpm at 4 °C for 15 min. Aliquots of supernatants were separated for biochemical analysis using Shimadzu spectrophotometer (UV 120-02).
For DNA fragmentation using DNA laddering assay, a small piece of brain tissue was placed in 1 ml of cold Hank’s balanced salt solution (HBSS) containing 20 mM EDTA and 10% DMSO frozen at − 80 °C until analysis. Brain specimens from all groups were collected and fixed in 4% neutral buffered formaldehyde for histopathological and immuno-histochemical examination.
Biochemical estimations
Determination of MDA concentration
The concentration of malondialdehyde (MDA), lipid peroxidation marker, was detected in brain homogenates of different groups spectrophotometrically at wavelength 535 nm where MDA reacts with the thiobarbituric acid in an acidic medium when incubated for 45 min at 95 °C (Nair and Turner 1984).
Determination of protein carbonyl level
Protein carbonyl level in brain homogenate reacts with 2, 4-dinitrophenylhydrazine (DNPH) forming 2, 4-dinitro phenylhydrazone where DNPH incubated with brain samples for 1 h followed by washing several times with the trichloroacetate and ethanol-ethyl acetate mixture then redissolve 2, 4-dinitro phenylhydrazone in guanidine hydrochloride (Levine et al. 1990). The concentration of protein in brain homogenates was determined using bovine serum albumin as standard (Lowry et al. 1951).
Determination of reduced glutathione content
The level of reduced glutathione (GSH) was quantified in brain homogenates using 0.3 M disodium phosphate solution, 5, 5 dithiobis 2-nitrobenzoic acid (DTNB), and precipitating solution. In brief, DTNB is a disulfide chromogen that is readily reduced by sulfhydryl compounds to an intensely yellow compound. The absorbance of the reduced chromogen measured at 412 nm and directly proportional to GHS levels (Beutler 1969).
Determination of total ATP level
Rat brain ATP content measured according to instructions of Rat Adenosine Triphosphate (ATP) ELISA kit (Cat. No: KT-59182) obtained from KAMIYA BIOMEDICAL COMPANY). The yellow color produced was measured at wavelength 450 nm (Cailla et al. 1982).
Determination of glutathione reductase activity (GR)
The activity of glutathione reductase reduces oxidized glutathione to reduced form (GR; EC 1.8.1.7) in presence of NADPH that oxidized into NADP+. The decrease in absorbance at 340 nm was determined in brain homogenates according to Goldberg and Spooner (1988).
Determination of lactate dehydrogenase and acetyl cholinesterase
The activities of LDH in brain homogenates were measured according to the method of Cabaud et al. (1958). The reduction of pyruvate to lactate in presence of nicotinamide adenine dinucleotide reduced (NADH) is the principle of LDH activity where the rate of NADH consumed is proportional to catalytic activity of LDH at 340 nm. AChE in brain homogenates that were measured according to the method of Ellman et al. (1961) depends on a yellow color compound produced as a result of reaction between thiocholine and dithiobisnitrobenzoate.
Determination of 8-hydroxy-2′-deoxyguanosine level
Levels of 8-hydroxy-2′-deoxyguanosine in brain homogenate were measured using commercial ELISA kit obtained from Abnova, catalog number KA0444.
Reverse transcription polymerase chain reaction of apoptosis-related genes
Total RNA was extracted from 100 mg brain tissues using QIAzol reagents for 5 min. Then, 200 μl chloroform was added to each sample. Following 2-min incubation at room temperature, samples were centrifuged at 12,000 rpm for 15 min at 4 °C. The aqueous phase is then transferred to a fresh tube and mixed with absolute ethanol. The sample was then loaded on RNeasy Mini Spin column and centrifuged at 13,000×g for 30 s at room temperature. After repeated washes in RWT and RPE, RNA pellets were eluted with 50 μl diethylpyrocarbonate-treated water. RNA quality was assessed by estimated 260/280 nm ratio and a ratio of 1.8 was considered acceptable. Complementary DNA (cDNA) was synthesized as previously reported (Hussein and Ahmed 2016). In brief, a total volume of 20 μl reverses transcription reaction mixture containing random hexamer primers, dNTPs, MgCl2, and finally reverse transcriptase enzyme. The mixture was incubated at 42 °C for 15 min, heated to 99 °C for 5 min, and maintained at 5 °C for 5 min using a 2720 thermocycler (Applied Biosystems, USA). One microgram of each cDNA was subjected to PCR amplification in a total volume of 50 μl using PCR Master Mix (Fermentas, Cairo, Egypt) and gene specific primers. The GeneBank™ accession numbers of p-53, Bax, caspase-3, and Bcl-2 are NM_030989, NM_017059, NM_012922, and NM_016993, respectively. The PCR reaction conditions and primer sequences are given in Table 1 with an initial denaturation step at 95 °C for 3 min and a final extension step at 72 C for 10 min using a 2720 thermocycler (Applied Biosystems, USA). β-actin was used as a housekeeping gene for PCR with accession number BC063166, and their product size was 277 bp. A single major band for each gene was detected by electrophoresis on a 1.5% agarose/ethidium bromide. The product size for p-53, Bax, caspase-3, and Bcl-2 were 106, 136, 412, and 446 bp, respectively. The intensity of the bands was compared with those collected from the control group using the public domain NIH Image program (National Institutes of Health, Bethesda, Maryland). The transcriptional levels were expressed as the mean ± standard error (SE).
DNA fragmentation ladder assay
Brain samples from all groups were used for DNA fragmentation assay according to the method described by Bortner et al. (1995). Frozen samples were homogenized with 0.5% SDS extraction buffer (1 M Tris/base, 0.5 M EDTA, NaCl 5 M, SDS 2.5 g, pH 8.0) then added 50 μl of proteinase-K solution (10 mg/ml), incubate tubes at 55 °C with occasional vigorous mixing, remove tubes from incubator then add 0.7–0.8 ml (phenol/chloroform/isoamyl alcohol in 25:24:1) and vortex samples for 2–5 s. Centrifuge samples at 12,000 rpm for 3–5 min, remove 400–500 μl aqueous layer for each sample in the new tube. Add 40–50 μl 3 M sodium acetate pH 5.3 to each tube. Add ethanol 100%, precipitated DNA allowed to set at − 20 °C overnight. Centrifuge at 12,000 rpm for 20 min. Remove the supernatant and add 50 μl of tries EDTA buffer overnight till complete dissolving. Run samples on electrophoresis using 1.2% agarose and 50 V; the gel was stained using ethidium bromide. Samples were analyzed using image analyses software.
Histopathological and immunohistochemical investigation
Brain specimens (right cerebral cortices) were collected from rats of different groups then fixed in 10% neutral buffered formalin solution. Five-micron thick paraffin sections were prepared, stained with hematoxylin and eosin for histopathological examination (Gamble and Wilson 2008). Consecutive sections were prepared for immunohistochemical investigation for detection of apoptotic markers (caspase 3 and Bcl2) positive cells by the avidine–biotin–peroxidase (ABC) method as previously described by Shi et al. (1991). Negative control sections were applied by incubating with phosphate-buffered saline 0.01 M instead of the primary antibodies. The following primary monoclonal antibodies were used: anti-caspase-3 antibody (ab52181) (Abcam Inc.) and human/mouse Bcl-2 (R&D Systems, Inc). All tissue sections were then observed by light microscopy. Sample scores in IHC examination were calculated by assignment to the percentage of positive cells per each three high power fields (HPF) at 40× magnification as following: score 0 = < 10%, score 1 + = 10–25%, score 2 + = 25–50%, score 3 + = 50–75%, and score 4 + => 75%.
Statistical analysis
Data were analyzed for a statistical significance between the control and treated groups with an analysis of variance (one-way ANOVA) with the SPSS 10.1 computer program (SPSS) followed by Duncan’s multiple range test. P values < 0.05 were used as the criterion for significance. Data were expressed as means ± SE.
Results
Effects on biochemical markers in brain homogenates
Tables 2 and 3 illustrate the neurotoxic impact of K2Cr2O7 oral exposure along with VC pre and/or concurrent supply. K2Cr2O7 significantly increased lipid peroxidation evidenced by elevated MDA levels in brain homogenates which was significantly compared to the (C) group. However, VC administration significantly decreased the elevated MDA levels, and the improved effect was significant in VC + Cr pro and co-treated group than VC + Cr co-treated group compared to the C group as shown in Table 2. In regard to protein carbonyl (PCO), a marker of protein oxidative damage, K2Cr2O7 exposure significantly increased PCO levels compared to the C group. Meanwhile, the VC supply improved the elevated levels which were more significant in VC + Cr pro and co-treated group than VC + Cr co-treated group, and the amelioration was non-significant in VC + Cr co-treated group compared to the C group as shown in Table 2. Concerning total GSH content, K2Cr2O7 exhibits a significant decrease in GSH levels in brain homogenates of (Cr) group compared to the C group. VC supply enhanced the decreased levels of GSH content and the better effect was observed in VC + Cr pro and co-treated group more significantly than VC + Cr co-treated compared to the C group as shown in Table 2. In regard to total ATP level in brain homogenates, Cr group showed a significant decrease compared to the C group. VC supply enhanced the decreased levels of total ATP. Nevertheless, the improvement was non-significant between VC + Cr pro and co-treated group and VC + Cr co-treated group compared to the C group as shown in Table 2. The obtained data in this study about GR revealed a significant decrease in Cr-administered groups compared to the C group as shown in Table 2. Nevertheless, this activity is improved in VC-treated groups especially in VC + Cr pro and co-treated group more significantly than VC + Cr co-treated group compared to the C group.
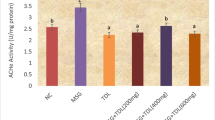
Belonging to the activity of LDH enzyme, there was a significant increase in Cr group compared to the C group. Meanwhile, VC administration decreased the elevated levels but the decrease was non-significant between VC + Cr pro and co-treated group and VC + Cr co-treated group compared to the C group as shown in Table 3. Concerning AChE activity in brain homogenates, Cr group showed a significant decrease compared to the C group. Nevertheless, VC + Cr pro and co-treated group showed more significant improvement than VC + Cr co-treated group in regard to the C group as shown in Table 3. Brain protein concentration in Cr-exposed rats showed a significant decrease compared to the C and VC groups. While VC supply significantly reversed its levels nearly to the normal compared to the C group which is more significantly in VC + Cr pro and co-treated group than VC + Cr co-treated group as shown in Table 3.
Brain 8oH-2̀dG levels a marker of oxidative DNA damage were significantly increased in Cr-exposed group compared to the C group. Meanwhile, the VC administration improved the elevated levels significantly in VC + Cr pro and co-treated group more than VC + Cr co-treated group compared to the C group as shown in Fig. 1.
Effects on p-53, Bax, caspase-3, and Bcl-2 mRNA expression in brain tissue
The transcriptional levels of p-53, Bax, and caspase-3 mRNA expression in brain tissue of Cr-exposed rats revealed significantly up-regulated expression levels compared to the C group. However, in VC pro and/or co-treated groups, expression levels were down-regulated compared to the Cr-exposed group. And the down-regulation was more significant in VC + Cr pro and co-treated group than VC + Cr co-treated group as shown in Fig. 2a–c. On the other hand, the expression level of Bcl-2 was significantly decreased in Cr group compared to the C group. However, VC supply succeeds to improve the mRNA expression levels to those of C group. Also, Bcl-2 expression was significantly up-regulated in VC + Cr pro and co-treated group more than VC + Cr co-treated group Fig. 2d. Thus, VC modulates mRNA expression patterns of p-53, Bax, caspase-3, and Bcl-2.
Effects of K2Cr2O7 oral exposure (25 mg/kg b.wt) along with VC (120 mg/kg b.wt) pro and/or concurrent supply on male rat brain p53, Bax, caspase-3, and Bcl2 mRNA expression manner showing electrophoretic bands corresponding to amplification of different apoptotic gene expression (upper) and β-actin (lower). Each bar carrying different letters was significantly different (P < 0.05)
Effects of Cr and vitamin C pro and/or Co administration on brain DNA fragmentation
Results are shown in Fig. 3. There was a very low or undetectable DNA laddering (DNA fragmentation) in the neurocytes of the control rats (C) and (VC) treated group (lanes 1, 2, 3 and lanes 4, 5, 6), respectively. The DNA intact band appears to be condensed near the application point with no DNA smearing suggesting no DNA fragmentation. On the other hand, the Cr-exposed group showed massive DNA fragmentations which appear as DNA laddering (lanes 7, 8, 9). In Cr + VC co-treated group, apoptotic bands were observed with slight shadow laddering area (lanes 10, 11, 12) while in VC + Cr pro and co-treated group, no apoptotic bands were observed (lanes 13, 14, 15).
Gel stained with ethidium bromide showing, M: standard marker (50 bp Plus DNA ladder). Lanes 1, 2, 3: C group showed one intact band of DNA with slight shadowing. Lanes 4, 5, 6: VC group showed 2 thin bands of DNA the first at 10,000 and at 3000 bp. Lanes 7, 8, 9: Cr group showed a long area of shadowing along the lane in between 10,000 and 250 bp. Lanes 10, 11, 12: VC + Cr co-treated group showed one intact band of DNA with slight shadowing till the level of 3000 bp. Lanes 13, 14, 15: VC + Cr pro and co-treated group showed one intact band of DNA at the level of 10,000 bp
Histopathological and immunohistochemical investigation
Examination of the brain tissue sections from the control and VC-administered group revealed normal histological architecture and structure of the neurons and neuropil (Fig. 4a, b, respectively). The brain tissue of Cr-exposed rats showed severe vacuolation of the neurons and intercellular edema appeared as spongiosis of the neuropil (Fig. 4c). Moreover, moderate congestion of the cerebral capillaries was observed in the same group (Fig. 4d).
Photomicrograph of the cerebral cortex at the mid-infundibular level (H&E bar = 50 μm): a C and b VC groups showing normal structure of the neurons; c Cr group showing severe vacuolation of the neurons (arrowheads) and spongiosis of the neuropil; d Cr group showing moderate congestion of the cerebral capillaries (arrowheads); e VC + Cr co-treated group showing moderate vacuolation of the neuropil with vacuolation of few neurons (arrowheads); f VC + Cr pro and co-treated group showing normal histological structures of the majority of neurons with slight spongiosis of the neuropil
Examined brain sections from VC + Cr co-treated group revealed moderate vacuolation of the neuropil with vacuolation of few neurons (Fig. 4e). While in VC + Cr pro and co-treated group, brain tissue that showed restoration of the normal histological appearance in the majority of neurons with slight spongiosis of the neuropil was detected (Fig. 4f).
Immunohistochemical (IHC) staining of the brain tissue with anti-caspase 3 antibodies of control and VC received group (Fig. 5a, b) revealed the absence of immunostaining in all neurons and the surrounding neuropil and was scored = 0. In the Cr-exposed group, the majority of neurons, glial cells, and neuropil showed positive immunostaining and were scored 3+ (Fig. 5c). Examined sections of VC + Cr co-treated group revealed positive immunolabeling of the neuropil and few neurons with the score 2+ (Fig. 5d). Brain sections of VC + Cr pro and the co-treated group showed weak immunostaining of the neuropil and very few neurons and were scored 1+ (Fig. 5e). IHC staining of the brain tissue with the anti-Bcl2 antibody of control and VC received group (Fig. 6a, b) revealed positive immunostaining of neuropil were scored 2+. In the Cr-exposed group, slight immunostaining of the neuropil and rare neurons was scored 1+ (Fig. 6c). Brain sections of VC + Cr co-treated group revealed positive immunostaining of the neuropil and few neurons with the score 3+ (Fig. 6d). In VC + Cr pro and co-treated group, neuron and glial cells showed increased positive immunolabeling and were scored 4+ (Fig. 6e).
Immunohistochemical staining of the cerebral cortex at the mid-infundibular level tissue with anti-caspase 3 antibody (bar = 50 μm); a C and b VC showing absence of immunostaining in all neurons and the surrounding neuropil (score: 0); c Cr showing positive immunolabeling of the majority of neurons and neuropil (arrowheads) (score: 3+); d VC + Cr co-treated showing positive immunostaining of few neurons (arrowheads) and absence of the labeling in the neuropil (score: 2+); e VC + Cr pre and co-treated showing positive immunostaining of few neurons (arrowheads) and absence of the labeling in the neuropil (score: 1+)
Immunohistochemical staining of the cerebral cortex at the mid-infundibular level tissue with anti-Bcl2 antibody (bar = 50 μm); a C and b VC showing positive immunostaining in all neurons and the surrounding neuropil (score = 2+); c Cr showing slight immunolabeling of the neurons and neuropil (arrowheads) (score: 1+); d VC + Cr co-treated showing positive immunostaining of the neuropil and few neurons (arrowheads) (score: 3+); e VC + Cr pro and co-treated group showing increased positive immunostaining of the neuropil (arrowheads) and few neurons (score: 4+)
Discussion
Few studies on the effects of Cr IV on the functional and structural integrity of the mammalian brain are present. Accordingly, our present design extends the previous research data on apoptotic and carcinogenic effects of hexavalent chromium oral administration, but specifically on rat brain, and advocated the effects of exogenous potentiating oral doses of ascorbic acid in the treatment of Cr-induced apoptosis. Brain, a mammalian organ, is frequently targeted by oxidative injury and resultant destructive lipid peroxidation, protein oxidation, and DNA damage owing to its high polyunsaturated fatty acid content, little antioxidant protection, and high iron contents (Jayaraman et al. 2008).
Obtained results revealed that oral intake of K2Cr2O7 resulted in significant decrease in GSH content, GR activity, ATP levels, and AChEs activity while MDA concentration, 8oH-2̀dG, protein carbonyl levels, DNA fragmentation, and LDH enzyme were significantly increased. According to previous studies (Bagchi et al. 2002; Wang et al. 2006; Jin et al. 2015), Cr can induce oxidative stress, lipid peroxidation, DNA damage, and apoptotic cell death in different experimental models. Cr is a transition group metal that has several oxidation states; its structural similarity with sulfates and phosphate ions facilitates its cellular entry through non-specific anion channels (Salnikow and Zhitkovich 2007) disrupting intracellular oxidation/reduction equilibrium. Endogenous antioxidants including ascorbic acid and cysteine effectively reduce chromium (VI) to different intermediates (V), (IV), and chromium (III) resulting in generation of ROS in many tissues including brain (Nudler et al. 2009). Cr VI is also reduced by GR, a flovoenzyme, to Cr V yielding superoxide anion radical which dismutated further to hydrogen peroxide radical. Additionally, Cr V ions and hydrogenperoxide radicals are involved in Fenton-like reaction producing more deterimental hydroxyl radicals. The total generated ROS spectrum damaging cellular components leading to lipid peroxidation, protein and DNA damage, affect the levels and functions of redox sensitive signaling molecules, such as p53, disturbing the cell signaling and gene expression systems, and/or induce apoptosis.
GSH content is significantly depleted in Cr-administered rats. Previous data acknowledged that Cr (VI) generally depletes GSH in many systems (O’Brien et al. 2003; Subramanian et al. 2006; Lalaouni et al. 2007). This may be attributed to the intracellular reduction of Cr by several molecules/compounds such as ascorbates, glutathione (GSH) or may be due to alteration in activity of enzymes involved its regeneration which is observed in obtained results of the present study including decreased GR enzyme activity (one of the main enzymatic antioxidant targeted potentially by heavy metals toxicities inducing oxidative stress). These results are in disagreement with that obtained by García-Niño et al. (2015) who mentioned that K2Cr2O7 failed to induce oxidative injury represented by unchanged levels of malondialdehyde (MDA), glutathione (GSH), and the activity of GSH-related enzymes in different rat organs including the brain. This may be attributed to the different route, dose, and duration of exposure. Also, results are in disagreement with those of Quinteros et al. (2008) who mentioned that Cr (VI) increased GSH content in anterior pituitary gland cells. In our study, depleted GSH may result as a protective antioxidant mechanism versus Cr-induced ROS generation. It has been shown that GSH depletion leads to cell death in different organs (Chandra et al. 2000; O’Brien et al. 2003). AChEs activity is a vital biomarker used to evaluate exposure to environmental toxins including pesticides and recently used to assess heavy metal neurotoxicity (Tsangaris et al. 2007). AChEs activity was significantly inhibited in Cr-exposed rats. These results are in concordance with that obtained by Kim and Kang (2016) which indicates that Cr exposure affects the cholinergic signaling. Therefore, the high level of dietary Cr exposure may significantly influence mammalian neurotoxicity. Elevated LDH levels indicate cellular damage in the brain which is concordant with Krim et al. (2013). After membrane lipid peroxidation, evidenced by increased MDA, the permeability of neuronal membranes is increased leading to enzyme leakage from the cytosol. Decreased ATP content may be due to Cr (VI)-induced energy metabolism disorders. Few studies have investigated the association between ROS and dysfunctional mitochondrial metabolism which could be due to mitochondrial-mediated apoptosis that resulted from oxidative stress inducing xenobiotics (Son et al. 2010). Inside the cells, Cr (VI) is reduced and ROS is generated which attack DNA and injure its integrity yielding DNA structural lesions, dysfunctional DNA replication, and transcription and disruption of regulatory genes responsible for the balance of cell survival and cell death, which incriminated in Cr (VI)-induced apoptosis. Cr (VI) enhanced ROS production, DNA fragmentation, apoptotic cell death, and altered gene expression (Bagchi et al. 2001; Banu et al. 2011).
Sensitive changes after pollutant exposure occur firstly at the transcription level (Viarengo et al. 2007). Increased ROS levels act as a signal messenger and activate oxidative stress transcription factors such as p53 (Haupt et al. 2003). Previous studies showed that Cr accumulates in the anterior pituitary gland cells inducing oxidative stress and apoptosis (Quinteros et al. 2008, 2007). P 53 is activated by Cr (VI) and ROS are needed for its activation. There is a close correlation between ROS generation, DNA damage, and p53 activation. Thus, ROS are required in the p 53-dependent apoptotic pathway. It is obviously clear that Cr VI initiates oxidative stress and cytotoxicity in the brain of exposed rats which is evidenced by obtained data in current study (deletion of GSH, decreased GR, increased MDA, PCO, and oxidatively damaged DNA 8OH2dG), ending finally with apoptotic consequences. These results showed that Cr VI induced DNA damage and p 53-dependent manner as mentioned by O’ Brien et al. (2003). Prolonged and persistent p53 activation leads to death signaling through changes of the Bcl-2 protein family (Galluzzi et al. 2008; Kook et al. 2007; Lee et al. 2008). The down-regulated Bcl2 expression is in agreement with that obtained by (Wang et al. 2006 and Son et al. (2010). In the brain, Bcl-2 protects against apoptotic neuronal death (Zhong et al. 1993), and it is one of the major anti-apoptotic proteins integrated into the mitochondrial membrane. A notable result of this study is that K2Cr2O7 reduces anti-apoptotic Bcl-2 expression in rat brain indicating the involvement of mitochondrial stress in Cr (VI)-induced apoptosiswhich evidenced by depleted ATP levels, seen in our study, in brain tissues of Cr VI exposed rats. Up-regulated Bax gene expression may be attributed to the increased p 53 activation which induced Bax expression and suppress Bcl-2 expression. Thus, decreased Bcl-2 and induced Bax gene expression may be attributed to activated p 53 induced by Cr VI (Wang et al. 2006). Regarding the activated caspase-3 expression, the last executer of intracellular apoptosis, obtained results showed that oral Cr VI induced caspase-3 activation. These results are in accordance with those observed by Wang et al. (2006). This may be due to mitochondrial dysfunction, decreased Bcl-2, and elevated Bax levels leading to loss of mitochondrial membrane potential and release of cytochrome c which is vital for activation of caspase-3, a key factor in Cr VI-induced apoptosis. Increased levels of oxidative DNA damage evidenced by increased 8OH2dG in Cr VI-exposed rat brain will delay cell cycle regression transiently until damaged DNA is repaired. However, if the DNA damage was severe and not repaired, cell undergoes apoptosis which is evidenced in the current study by increase in the fragmented DNA in DNA laddering assay.”
It has been recommended that intake of antioxidants would assist in ameliorating deleterious effects induced by oxidative stress. VC, a nutritional antioxidant, exerts its protective potential against all deterimental toxic effects induced by Cr VI in the present work. Topical VC is used against Cr-induced dermatitis (Bradberry and Vale 1999) and parenteral VC reduced sodium dichromate nephrotoxicity in animals. However, info on the efficacy of oral VC in Cr poisoning is still insufficient. VC is the main reductant of Cr (VI) in animals and cell cultures (Kesinger and Stevens 2009) interrupting radical chain reactions in biological membranes (Stearns et al. 1995). The efficacy of VC pre and concurrent treatment more than concurrent treatment only is due to accelerated extracellular VC reduction of more readily absorbed Cr (VI) to less toxic Cr (III) prior to crossing cellular membranes thus decreasing its induced consequences. Our study confirmed that oral VC pre and concurrent administration counteract K2Cr2O7 toxicity than concurrent treatment only.
References
Bagchi D, Bagchi M, Stohs SJ (2001) Chromium (VI)-induced oxidative stress, apoptotic cell death and modulation of p53 tumor suppressor gene. Mol Cell Biochem 222:149–158
Bagchi D, Stohs SJ, Downs BW, Bagchi M, Preuss HG (2002) Cytotoxicity and oxidative mechanisms of different forms of chromium. Toxicology 180:5–22
Banu SK, Stanley JA, Lee J, Stephen SD, Arosh JA, Hoyer PB, Burghardt RC (2011) Hexavalent chromium-induced apoptosis of granulosa cells involves selective sub-cellular translocation of Bcl-2 members, ERK1/2 and p53. Toxicol Appl Pharmacol 251:253–266
Beutler E (1969) Effect of flavin compounds on glutathione reductase activity: in vivo and in vitro studies. J Clin Investig 48:1957–1966
Bortner CD, Oldenburg NB, Cidlowski JA (1995) The role of DNA fragmentation in apoptosis. Trends Cell Biol 5:21–26
Bradberry SM, Vale JA (1999) Therapeutic review: is ascorbic acid of value in chromium poisoning and chromium dermatitis? J Toxicol Clin Toxicol 37:195–200
Cabaud PG, Wróblewski F, Ruggiero V (1958) Colorimetric measurement of lactic dehydrogenase activity of body fluids. Am J Clin Pathol 30:234–236
Cailla H, De Kaouel CLB, Roux D, Delaage M, Marti J (1982) Monoclonal antibodies to 5′-triphospho-(2′-5′) adenyladenosine oligonucleotides. Proc Natl Acad Sci 79:4742–4746
Chandra J, Samali A, Orrenius S (2000) Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med 29:323–333
Costa M, Klein CB (2006) Toxicity and carcinogenicity of chromium compounds in humans. Crit Rev Toxicol 36:155–163
Dashti A, Soodi M, Amani N (2016) Cr (VI) induced oxidative stress and toxicity in cultured cerebellar granule neurons at different stages of development and protective effect of Rosmarinic acid. Environ Toxicol 31:269–277
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88IN191–88I9095
Galluzzi L, Morselli E, Kepp O, Tajeddine N, Kroemer G (2008) Targeting p53 to mitochondria for cancer therapy. Cell Cycle 7:1949–1955
Gamble M, Wilson I (2008) The hematoxylins and eosin. Theory Pract Histol Tech 6:121–134
García-Niño WR, Zatarain-Barrón ZL, Hernández-Pando R, Vega-García CC, Tapia E, Pedraza-Chaverri J (2015) Oxidative stress markers and histological analysis in diverse organs from rats treated with a hepatotoxic dose of Cr (VI): effect of curcumin. Biol Trace Elem Res 167:130–145
Goldberg DM, Spooner RJ (1988) 3.7 glutathione reductase. Enzymes 1:258
Haupt S, Berger M, Goldberg Z, Haupt Y (2003) Apoptosis-the p53 network. J Cell Sci 116:4077–4085
Hussein MM, Ahmed MM (2016) The Th1/Th2 paradigm in lambda cyhalothrin-induced spleen toxicity: the role of thymoquinone. Environ Toxicol Pharmacol 41:14–21
Jayaraman T, Kannappan S, Ravichandran M, Anuradha C (2008) Impact of Essentiale L on ethanol-induced changes in rat brain and erythrocytes. Singap Med J 49:320
Jin Y, Liu Z, Liu F, Ye Y, Peng T, Fu Z (2015) Embryonic exposure to cadmium (II) and chromium (VI) induce behavioral alterations, oxidative stress and immunotoxicity in zebrafish (Danio Rerio). Neurotoxicol Teratol 48:9–17
Kesinger NG, Stevens JF (2009) Covalent interaction of ascorbic acid with natural products. Phytochemistry 70:1930–1939
Kim J-H, Kang J-C (2016) Oxidative stress, neurotoxicity, and metallothionein (MT) gene expression in juvenile rock fish Sebastes Schlegelii under the different levels of dietary chromium (Cr 6+) exposure. Ecotoxicol Environ Saf 125:78–84
Kook S-H, Son Y-O, Chung S-W, Lee S-A, Kim J-G, Jeon Y-M, Lee J-C (2007) Caspase-independent death of human osteosarcoma cells by flavonoids is driven by p53-mediated mitochondrial stress and nuclear translocation of AIF and endonuclease G. Apoptosis 12:1289–1298
Krim M, Messaadia A, Maidi I, Aouacheri O, Saka S (2013) Protective effect of ginger against toxicity induced by chromate in rats. Ann Biol Clin 7(2):165–173
Kucharski H, Zajac J (2009) Handbook of vitamin C research: daily requirements, dietary sources and adverse effects. Nova Science Publishers, Inc., New York
Lalaouni A, Henderson C, Kupper C, Grant M (2007) The interaction of chromium (VI) with macrophages: depletion of glutathione and inhibition of glutathione reductase. Toxicology 236:76–81
Lee KB, Kim K-R, Huh T-L, Lee YM (2008) Proton induces apoptosis of hypoxic tumor cells by the p53-dependent and p38/JNK MAPK signaling pathways. Int J Oncol 33:1247–1256
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz A-G, Ahn B-W, Shaltiel S, Stadtman ER (1990) [49] determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Nair V, Turner GA (1984) The thiobarbituric acid test for lipid peroxidation: structure of the adduct with malondialdehyde. Lipids 19:804–805
Nickens KP, Patierno SR, Ceryak S (2010) Chromium genotoxicity: a double-edged sword. Chem Biol Interact 188:276–288
Nudler SI, Quinteros FA, Miler EA, Cabilla JP, Ronchetti SA, Duvilanski BH (2009) Chromium VI administration induces oxidative stress in hypothalamus and anterior pituitary gland from male rats. Toxicol Lett 185:187–192
O’Brien TJ, Ceryak S, Patierno SR (2003) Complexities of chromium carcinogenesis: role of cellular response, repair and recovery mechanisms. Mutat Res/Fundam Mol Mech Mutagen 533:3–36
Quinteros FA, Poliandri AH, Machiavelli LI, Cabilla JP, Duvilanski BH (2007) In vivo and in vitro effects of chromium VI on anterior pituitary hormone release and cell viability. Toxicol Appl Pharmacol 218:79–87
Quinteros FA, Machiavelli LI, Miler EA, Cabilla JP, Duvilanski BH (2008) Mechanisms of chromium (VI)-induced apoptosis in anterior pituitary cells. Toxicology 249:109–115
Qureshi IZ, Mahmood T (2010) Prospective role of ascorbic acid (vitamin C) in attenuating hexavalent chromium-induced functional and cellular damage in rat thyroid. Toxicol Ind Health 26:349–359
Rekha C, Poornima G, Manasa M, Abhipsa V, Devi P, Kumar V, Kekuda P (2012) Ascorbic acid, total phenol content and antioxidant activity of fresh juices of four ripe and unripe citrus fruits. Chem Sci Trans 1:303–310
Salnikow K, Zhitkovich A (2007) Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: nickel, arsenic, and chromium. Chem Res Toxicol 21:28–44
Shi S-R, Key ME, Kalra KL (1991) Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem 39:741–748
Son Y-O, Hitron JA, Wang X, Chang Q, Pan J, Zhang Z, Liu J, Wang S, Lee J-C, Shi X (2010) Cr (VI) induces mitochondrial-mediated and caspase-dependent apoptosis through reactive oxygen species-mediated p53 activation in JB6 Cl41 cells. Toxicol Appl Pharmacol 245:226–235
Stearns DM, Kennedy LJ, Courtney KD, Giangrande PH, Phieffer LS, Wetterhahn KE (1995) Reduction of chromium (VI) by ascorbate leads to chromium-DNA binding and DNA strand breaks in vitro. Biochemistry 34:910–919
Subramanian S, Rajendiran G, Sekhar P, Gowri C, Govindarajulu P, Aruldhas MM (2006) Reproductive toxicity of chromium in adult bonnet monkeys (Macaca Radiata Geoffrey). Reversible oxidative stress in the semen. Toxicol Appl Pharmacol 215:237–249
Tamari Y, Nawata H, Inoue E, Yoshimura A, Yoshii H, Kashino G, Seki M, Enomoto T, Watanabe M, Tano K (2013) Protective roles of ascorbic acid in oxidative stress induced by depletion of superoxide dismutase in vertebrate cells. Free Radic Res 47:1–7
Trzeciak A, Kowalik J, Malecka-Panas E, Drzewoski J, Wojewódzka M, Iwanenko T, Blasiak J (2000) Genotoxicity of chromium in human gastric mucosa cells and peripheral blood lymphocytes evaluated by the single cell gel electrophoresis (comet assay). Med Sci Monit 6:24–29
Tsangaris C, Papathanasiou E, Cotou E (2007) Assessment of the impact of heavy metal pollution from a ferro-nickel smelting plant using biomarkers. Ecotoxicol Environ Saf 66:232–243
Tsao DA, Tseng WC, Chang HR (2011) The expression of RKIP, RhoGDI, galectin, c-Myc and p53 in gastrointestinal system of Cr (VI)-exposed rats. J Appl Toxicol 31:730–740
Viarengo A, Lowe D, Bolognesi C, Fabbri E, Koehler A (2007) The use of biomarkers in biomonitoring: a 2-tier approach assessing the level of pollutant-induced stress syndrome in sentinel organisms. Comp Biochem Physiol Part C: Toxicol Pharmacol 146:281–300
Wang X-F, Xing M-L, Shen Y, Zhu X, Xu L-H (2006) Oral administration of Cr (VI) induced oxidative stress, DNA damage and apoptotic cell death in mice. Toxicology 228:16–23
Wartelle LH, Marshall WE (2005) Chromate ion adsorption by agricultural by-products modified with dimethyloldihydroxyethylene urea and choline chloride. Water Res 39:2869–2876
Wilbur S, Ingerman L, Citra M, Osier M, Wohlers D (2000) Toxicological profile for chromium. US Department of Health and Human Services. Public health service, Agency for Toxic Substances and Disease Registry, Washington DC, pp 1–419
Zhong L-T, Sarafian T, Kane DJ, Charles AC, Mah SP, Edwards RH, Bredesen DE (1993) bcl-2 inhibits death of central neural cells induced by multiple agents. Proc Natl Acad Sci 90:4533–4537
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All the experimental protocol is endorsed by the Approval of Ethics Committee of Faculty of Veterinary Medicine, Zagazig University, in accordance with the guiding principles of the National Institutes of Health (NIH) for the Care and Use of Laboratory Animals.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Abu Zeid, E.H., Hussein, M.M.A. & Ali, H. Ascorbic acid protects male rat brain from oral potassium dichromate-induced oxdative DNA damage and apoptotic changes: the expression patterns of caspase-3, P 53, Bax, and Bcl-2 genes. Environ Sci Pollut Res 25, 13056–13066 (2018). https://doi.org/10.1007/s11356-018-1546-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1546-9