Abstract
Purpose
In recent years, considerable progress has been made in the use of gallium-68 labeled receptor-specific peptides for imaging oncologic diseases. The objective was to examine the stability and pharmacokinetics of [68Ga]NODAGA and DOTA-peptide conjugate targeting VPAC [combined for vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating peptide (PACAP)] receptors on tumor cells.
Procedures
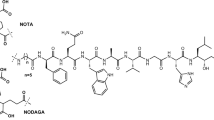
A VPAC receptor-specific peptide was chosen as a model peptide and conjugated to NODAGA and DOTA via solid-phase synthesis. The conjugates were characterized by HPLC and MALDI-TOF. Following Ga-68 chelation, the radiochemical purity of Ga-68 labeled peptide conjugate was determined by radio-HPLC. The stability was tested against transmetallation using 100 nM Fe3+/Zn2+/Ca2+ ionic solution and against transchelation using 200 μM DTPA solution. The ex vivo and in vivo stability of the Ga-68 labeled peptide conjugate was tested in mouse plasma and urine. Receptor specificity was determined ex vivo by cell binding assays using human breast cancer BT474 cells. Positron emission tomography (PET)/X-ray computed tomography (CT) imaging, tissue distribution, and blocking studies were performed in mice bearing BT474 xenografts.
Results
The chemical and radiochemical purity was greater than 95 % and both conjugates were stable against transchelation and transmetallation. Ex vivo stability at 60 min showed that the NODAGA-peptide-bound Ga-68 reduced to 42.1 ± 3.7 % (in plasma) and 37.4 ± 2.9 % (in urine), whereas the DOTA-peptide-bound Ga-68 was reduced to 1.2 ± 0.3 % (in plasma) and 4.2 ± 0.4 % (in urine) at 60 min. Similarly, the in vivo stability for [68Ga]NODAGA-peptide was decreased to 2.1 ± 0.2 % (in plasma) and 2.2 ± 0.4 % (in urine). For [68Ga]DOTA-peptide, it was decreased to 1.4 ± 0.3 % (in plasma) and 1.2 ± 0.4 % (in urine) at 60 min. The specific BT474 cell binding was 53.9 ± 0.8 % for [68Ga]NODAGA-peptide, 25.8 ± 1.4 % for [68Ga]-DOTA-peptide, and 18.8 ± 2.5 % for [68Ga]GaCl3 at 60 min. Inveon microPET/CT imaging at 1 h post-injection showed significantly (p < 0.05) higher tumor to muscle (T/M) ratio for [68Ga]NODAGA-peptide (3.4 ± 0.3) as compared to [68Ga]DOTA-peptide (1.8 ± 0.6). For [68Ga]GaCl3 and blocked mice, their ratios were 1.5 ± 0.6 and 1.5 ± 0.3 respectively. The tissue distributions data were similar to the PET imaging data.
Conclusion
NODAGA is superior to DOTA in terms of radiolabeling kinetics. The method of radiolabeling was reproducible and yielded higher specific activity. Although both agents have relatively low in vivo stability, PET/CT imaging studies delineated BC tumors with [68Ga]NODAGA-peptide, but not with [68Ga]DOTA-peptide.






Similar content being viewed by others
References
Al-Nahhas A, Win Z, Szyszko T et al (2007) Gallium-68 PET: a new frontier in receptor cancer imaging. Anticancer Res 27:4087–4094
Banerjee SR, Pullambhatla M, Byun Y et al (2010) [68Ga]-labeled inhibitors of prostate-specific membrane antigen (PSMA) for imaging prostate cancer. J Med Chem 53:5333–5341
Ambrosini V, Campana D, Tomassetti P et al (2011) PET/CT with [68Ga]gallium-DOTA-peptides in NET: an overview. Eur J Radiol 80:116
Baum RP, Kulkarni HR, Carreras C (2012) Peptides and receptors in image-guided therapy: theranostics for neuroendocrine neoplasms. Semin Nucl Med 42:190–207
Weiner RE, Thakur ML (2005) Radiolabeled peptides in oncology: role in diagnosis and treatment. BioDrugs 19:145–163
Mojtahedi A, Thamake S, Tworowska I, Ranganathan D, Delpassand ES (2014) The value of 68Ga-DOTATATE PET/CT in diagnosis and management of neuroendocrine tumors compared to current FDA approved imaging modalities: a review of literature. Am J Nucl Med Mol Imaging 4:426–434
Notni J, Pohle K, Wester HJ (2012) Comparative gallium-68 labeling of TRAP-, NOTA-, and DOTA-peptides: practical consequences for the future of gallium-68-PET. EJNMMI Res 2:28
Pfob CH, Ziegler S, Graner FP, Köhner M, Schachoff S, Blechert B, Wester HJ, Scheidhauer K, Schwaiger M, Maurer T, Eiber M (2016) Biodistribution and radiation dosimetry of 68Ga-PSMA HBED CC-a PSMA specific probe for PET imaging of prostate cancer. Eur J Nucl Med Mol Imaging 43:1962–1970
Prasad V, Ambrosini V, Hommann M, Hoersch D, Fanti S, Baum RP (2010) Detection of unknown primary neuroendocrine tumours (CUP-NET) using 68Ga-DOTA-NOC receptor PET/CT. Eur J Nucl Med Mol Imaging 37:67–77
Prata MI (2012) Gallium-68: a new trend in PET radiopharmacy. Curr Radiopharm 5:142–149
Altai M, Strand J, Rosik D, Selvaraju RK, Eriksson Karlström A, Orlova A, Tolmachev V (2013) Influence of nuclides and chelators on imaging using affibody molecules: comparative evaluation of recombinant affibody molecules site-specifically labeled with 68Ga and 111In via maleimido derivatives of DOTA and NODAGA. Bioconjug Chem 24:1102–1109
Trencsenyi G, Denes N, Nagy G et al (2017) Comparative preclinical evaluation of [68Ga]-NODAGA and [68Ga]-HBED-CC conjugated procainamide in melanoma imaging. J Pharm Biomed Anal 139:54–64
Kumar P, Tripathi SK, Chen CP, Mehta N, Paudyal B, Wickstrom E, Thakur ML (2016) Evaluation of a PACAP peptide analogue labeled with 68Ga using two different chelating agents. Cancer Biother Radiopharm 31:29–36
Tripathi S, Trabulsi EJ, Gomella L, Kim S, McCue P, Intenzo C, Birbe R, Gandhe A, Kumar P, Thakur M (2016) VPAC targeted (64)Cu-TP3805 positron emission tomography imaging of prostate cancer: preliminary evaluation in man. Urology 88:111–118
Thakur ML, Zhang K, Berger A, Cavanaugh B, Kim S, Channappa C, Frangos AJ, Wickstrom E, Intenzo CM (2013) VPAC receptors for imaging breast cancer: a feasibility study. J Nucl Med 54:1019–1025
Reubi JC, Laderach U, Waser B et al (2000) Vasoactive intestinal peptide/pituitary adenylate cyclase-activating peptide receptor subtypes in human tumors and their tissues of origin. Cancer Res 60:3105–3112
Zia H, Hida T, Jakowlew S, Birrer M, Gozes Y, Reubi JC, Fridkin M, Gozes I, Moody TW (1996) Breast cancer growth is inhibited by vasoactive intestinal peptide (VIP) hybrid, a synthetic VIP receptor antagonist. Cancer Res 56:3486–3489
Valdehita A, Carmena MJ, Bajo AM, Prieto JC (2012) RNA interference-directed silencing of VPAC receptor inhibits VIP effects on both EGFR and HER2 transactivation and VEGF secretion in human breast cancer cells. Mol Cell Endocrinol 348:241–246
Valdehita A, Bajo AM, Fernandez-Martinez AB et al (2010) Nuclear localization of vasoactive intestinal peptide (VIP) receptors in human breast cancer. Peptides 31:2035–2045
Moody TW, Gozes I (2007) Vasoactive intestinal peptide receptors: a molecular target in breast and lung cancer. Curr Pharm Des 13:1099–1104
Leyton J, Gozes Y, Pisegna J et al (1999) PACAP(6-38) is a PACAP receptor antagonist for breast cancer cells. Breast Cancer Res Treat 56:177–186
Schulz S, Rocken C, Mawrin C et al (2004) Immunocytochemical identification of VPAC, VPAC2, and PAC1 receptors in normal and neoplastic human tissues with subtype-specific antibodies. Clin Cancer Res 10:8235–8242
Reubi JC (1997) Regulatory peptide receptors as molecular targets for cancer diagnosis and therapy. Q J Nucl Med 41:63–70
Reubi JC (1995) Neuropeptide receptors in health and disease: the molecular basis for in vivo imaging. J Nucl Med 36:1825–1835
Asti M, De Pietri G, Fraternali A et al (2008) Validation of 68Ge/68Ga generator processing by chemical purification for routine clinical application of 68Ga-DOTATOC. Nucl Med Biol 35:721–724
Decristoforo C, Knopp R, von Guggenberg E, Rupprich M, Dreger T, Hess A, Virgolini I, Haubner R (2007) A fully automated synthesis for the preparation of [68Ga]-labelled peptides. Nucl Med Commun 28:870–875
Fani M, Andre JP, Maecke HR (2008) [68Ga]-PET: a powerful generator-based alternative to cyclotron-based PET radiopharmaceuticals. Contrast Media Mol Imaging 3:67–77
Zhang K, Aruva MR, Shanthly N, Cardi CA, Rattan S, Patel C, Kim C, McCue P, Wickstrom E, Thakur ML (2008) PET imaging of VPAC expression in experimental and spontaneous prostate cancer. J Nucl Med 49:112–121
Thakur ML, Devadhas D, Zhang K, Pestell RG, Wang C, McCue P, Wickstrom E (2010) Imaging spontaneous MMTVneu transgenic murine mammary tumors: targeting metabolic activity versus genetic products. J Nucl Med 51:106–111
Viola-Villegas N, Doyle R (2009) The coordination chemistry of 1,4,7,10-tetraazacyclododecane-N, N’, N”, N‴-tetraacetic acid (H4DOTA): structural overview and analyses on structure-stability relationships. Coord Chem Rev 253:1906–1925
Notni J, Steiger K, Hoffmann F, Reich D, Schwaiger M, Kessler H, Wester HJ (2016) Variation of specific activities of [68Ga]-Aquibeprin and [68Ga]-Avebetrin enables selective PET imaging of different expression levels of integrins alpha5beta1 and alphavbeta3. J Nucl Med 57:1618–1624
Asti M, Iori M, Capponi PC, Atti G, Rubagotti S, Martin R, Brennauer A, Müller M, Bergmann R, Erba PA, Versari A (2014) Influence of different chelators on the radiochemical properties of a 68-gallium labelled bombesin analogue. Nucl Med Biol 41:24–35
Gourni E, Demmer O, Schottelius M et al (2011) PET of CXCR4 expression by a 68Ga-labeled highly specific targeted contrast agent. J Nucl Med 52:1803–1810
Kang CM, Koo HJ, Choe YS, Choi JY, Lee KH, Kim BT (2014) 68Ga-NODAGA-VEGF121 for in vivo imaging of VEGF receptor expression. Nucl Med Biol 41:51–57
Mirzaei A, Jalilian AR, Aghanejad A, Mazidi M, Yousefnia H, Shabani G, Ardaneh K, Geramifar P, Beiki D (2015) Preparation and evaluation of 68Ga-ECC as a PET renal imaging agent. Nucl Med Mol Imaging 49:208–216
Persson M, Madsen J, Ostergaard S et al (2012) [68Ga]-labeling and in vivo evaluation of a uPAR binding DOTA- and NODAGA-conjugated peptide for PET imaging of invasive cancers. Nucl Med Biol 39:560–569
Pohle K, Notni J, Bussemer J, Kessler H, Schwaiger M, Beer AJ (2012) [68Ga]-NODAGA-RGD is a suitable substitute for 18F-Galacto-RGD and can be produced with high specific activity in a cGMP/GRP compliant automated process. Nucl Med Biol 39:777–784
Prinsen K, Cona MM, Cleynhens BJ, Li J, Vanbilloen H, Dyubankova N, Lescrinier E, Ni Y, Bormans GM, Verbruggen AM (2013) Synthesis and biological evaluation of [68Ga]-bis-DOTA-PA as a potential agent for positron emission tomography imaging of necrosis. Nucl Med Biol 40:816–822
Sun Y, Ma X, Zhang Z, Sun Z, Loft M, Ding B, Liu C, Xu L, Yang M, Jiang Y, Liu J, Xiao Y, Cheng Z, Hong X (2016) Preclinical study on GRPR-targeted 68Ga-probes for PET imaging of prostate cancer. Bioconjug Chem 27:1857–1864
Ujula T, Salomaki S, Virsu P et al (2009) Synthesis, [68Ga] labeling and preliminary evaluation of DOTA peptide binding vascular adhesion protein-1: a potential PET imaging agent for diagnosing osteomyelitis. Nucl Med Biol 36:631–641
Vilche M, Reyes AL, Vasilskis E, Oliver P, Balter H, Engler H (2016) 68Ga-NOTA-UBI-29-41 as a PET tracer for detection of bacterial infection. J Nucl Med 57:622–627
Domnanich KA, Müller C, Farkas R et al (2016) 44Sc for labeling of DOTA- and NODAGA-functionalized peptides: preclinical in vitro and in vivo investigations. EJNMMI Radiopharmacy Chem 1:8
Acknowledgements
We thank Kim Lee for providing technical assistance on this project.
Funding
The research, in part, was supported by NIH/NCI RO1CA157372 (MLT), NIH/NCI 1S10OD012406 (MLT), and NIH/NCI S10RR23709 (MLT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Mathew L. Thakur is consultant to NuView and Zevacor, Inc. No other potential conflicts of interest relevant to this article are reported.
Rights and permissions
About this article
Cite this article
Kumar, P., Tripathi, S.K., Chen, C.P. et al. Evaluating Ga-68 Peptide Conjugates for Targeting VPAC Receptors: Stability and Pharmacokinetics. Mol Imaging Biol 21, 130–139 (2019). https://doi.org/10.1007/s11307-018-1207-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-018-1207-x