Abstract
The origin of protein synthesis is one of the major riddles of molecular biology. It was proposed a decade ago that the ribosomal RNA evolved from an earlier RNA-replisome (a ribozyme fulfilling RNA replication) while transfer RNA (tRNA) evolved from a genomic replication origin. Applying these hypotheses, I suggest that protein synthesis arose for the purpose of segregating copy and template RNA during replication through the conventional formation of a complementary strand. Nascent RNA was scanned in 5′ to 3′ direction following the progress of replication. The base pairing of several tRNA-like molecules with nascent RNA released the replication intermediates trapped in duplex. Synthesis of random peptides evolved to fuel the turnover of tRNAs. Then the combination of replication-coupled peptide formation and the independent development of amino acid-specific tRNA aminoacylation resulted in template-based protein synthesis. Therefore, the positioning of tRNAs adjacent to each other developed for the purpose of replication rather than peptide synthesis. This hypothesis does not include either selection for useful peptides or specific recognition of amino acids at the initial evolution of translation. It does, however, explain a number of features of modern translation apparatus, such as the relative flexibility of genetic code, the number of proteins shared by the transcription and translation machines, the universal participation of an RNA subunit in co-translational protein secretion, ‘unscheduled translation’, and factor-independent translocation. Assistance of original ribosomes in keeping apart the nascent transcript from its template is still widely explored by modern bacteria and perhaps by other domains of life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In modern life, replication of nucleic acids operates through distinct but equally significant steps of the synthesis of complementary copies and the segregation of products. The importance of both synthesis and segregation capacities in the origin of life is well recognized (Campbell 1991; Joyce 2002). It was suggested that the ribosome has evolved from an earlier RNA-replisome, which originally synthesized RNA in sophisticated trinucleotide steps without making complementary molecules (Campbell 1991). This article explores the hypothesis that the process of protein synthesis evolved for the purpose of segregating the complementary products of conventional replication.
The ability of RNA to fulfill both genetic and catalytic functions gave rise the RNA world hypothesis for the origin of life (Gilbert 1986; Orgel 2003). Coexistence of genetic and catalytic activities among self-replicating RNA molecules, however, has obvious limitations inherent to the template-based RNA replication. First of all, the double-stranded helix formed by the template and copy strands is always more thermodynamically favorable than the separate single-stranded template and copy molecules. Secondly, no catalytic activity can be predicted for perfectly double-stranded RNA on the basis of the known structures of ribozymes. Although double-stranded RNA is able to fulfill a genetic function in some modern day viruses, it would be physiologically dead in the RNA world without an advanced protein-based replication system. Even in modern eukaryotes, the occasional appearance of double-stranded RNA activates stress responses, such as RNA interference and/or translational shutdown (Sledz and Williams 2004).
Relevant Features of the Modern Apparatus of Protein Biosynthesis
Protein synthesis is initiated by the binding of small ribosomal subunit to mRNA. Both prokaryotic and eukaryotic small ribosomal subunits have a markedly greater affinity to single-stranded RNA. Secondary structure in mRNA inhibits translation initiation or necessitates the assistance of auxiliary initiation factors. In contrast, translation elongation is substantially more resistant to secondary structures in mRNA. Moreover, the RNA helicase activity of the translating ribosomes has a potential to unwind rigid structures in mRNA and even displace a DNA oligonucleotide annealed to RNA template (Takyar et al. 2005). I propose that the RNA helicase/structure rearrangement activity was the original function of the translation apparatus.
Modern ribosomes are specialized for protein synthesis. However, the bacterial translational apparatus also participates in the regulation of gene expression by transcription attenuation. Attenuation operates through cotranscriptional synthesis of leader peptides; only one copy of peptide per mRNA is sufficient for regulation. The leader peptides are merely by-products of a regulated cotranscriptional RNA refolding (Yanofsky 2000). The purpose–tool relationships between RNA and peptide formation observed in the translation-assisted transcription attenuation can reflect the original function of polypeptide synthesis as an RNA-restructuring replication cofactor.
Another modern day example of regulation of nucleic acid synthesis through translational coupling is found in the in vitro replication of recombinant RQ RNA. The positive sense RNA bacteriophage Qβ replicates its genome via a negative strand intermediate made by the protein enzyme Qβ replicase. This RNA-dependent RNA polymerase peals the complementary strand off the template (Blumenthal and Carmichael 1979). Qβ replicase also efficiently replicates some noncoding satellite RNAs like RQ135 (Morozov et al. 1993). The combination of the Qβ replicase reaction with the Escherichia coli cell-free translation extract markedly enhanced replication of the recombinant RNA consisting of a protein coding sequence inserted into RQ135. The replication–translation coupling was also associated with the replication asymmetry; the coding (+)-strands are produced in large excess over the antisense (−)-strands. Translating ribosomes prevented the template and copy strands from annealing during coupled replication and translation, thus resulting in the positive effect of translational machinery on RNA replication. The effect was observed only with a recombinant RNA containing long unstructured coding region (Morozov et al. 1993). The extensive internal structure in naturally occurring templates of Qβ replicase (including RQ135, MDV-1, and phage genomic RNA) prevents the annealing of the template and copy molecules, in most cases, without the assistance of translating ribosomes, although a fixed proportion of each RNA is converted to the duplex form in the in vitro replication complexes (Priano et al. 1987).
Hypothesis
This work is based on two previously proposed models: the evolution of the ribosome from an earlier RNA replisome (Campbell 1991) and the genomic tag hypothesis, which states that in the RNA world, tRNA-like structures had been promoters of replication located at 3′ ends of genomic RNA and occasionally cut off mature catalytic RNA by RNaseP-like activity (Maizels and Weiner 1999). These tRNA-like structures could be aminoacylated in the process of marking replication-competent template RNAs and/or proper termination. Amino acids might facilitate the initiation of replication at the 3′ terminus of RNA templates (Orgel 1989) and even evolve to a promoter recognition element allowing discrimination between self-replicating RNA species. In any case, the genomic tag hypothesis postulated the emergence of free tRNA-like molecules aminoacylated with increasing specificity in the RNA world.
RNA Scanning in 5′ to 3′ Direction by a Small Ribosomal Subunit
RNA world should have resisted the coreplicational annealing of newly synthesized RNA with its template. Perhaps the ancient RNA replication process depended on the inherent ability of the catalytic RNA replisome to peel the product strands off their templates. Once separated, however, the replication products (in the vicinity of the end of the growing RNA chain) were free to reanneal upon losing contact with the replisome. This annealing could be prevented by fast coreplicational folding and refolding of the copy and template strands, respectively. The replication of modern bacteriophage Qβ is a good example of the initial nascent and template strand separation by the protein replicase followed by their maintenance in the single-stranded form by the internal secondary structure (Axelrod et al. 1991; Priano et al. 1987). Although internal structure in both the copy and the template RNA strands generally effectively competes with the alternative intermolecular annealing, this solution is not universal. A fixed proportion of replication complexes (which depends on the replicating RNA species) forms duplexes, resulting in a decreased replication rate and severely affecting the continual propagation of RNA populations (Axelrod et al. 1991; Priano et al. 1987). Moreover, some tertiary structures involving long-range interaction between remote regions of nascent RNA can be formed only after a delay. Bacteriophage MS2 uses such a folding delay to restrict the coreplicational translation initiation of its maturation gene to a short time period until the nascent positive RNA forms its final inactive structure (Poot et al. 1997). The replication of some phage Qβ satellite RNA involves the formation of metastable intermediate structures, which are subsequently replaced by the final versions (de Smit and van Duin 2003; Kramer and Mills 1981). Both the intermediate replication-associated structures and the folding delay can cause problems. Intermediate structures resist refolding at the unfavorable conditions whereas folding delays increase the probability of the nascent strand annealing with its template. Such an annealing process could be prevented if loosely structured regions remained blocked as long as possible (by binding to the RNA replisome, for example).
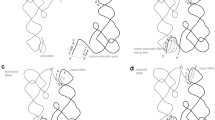
According to my current hypothesis, the process of scanning the single-stranded regions in the nascent RNA evolved as a molecular mechanism to prevent of the copy and the template strands from annealing upstream of the replication fork. The subunit of the RNA replisome fulfilling this scanning function became the ancestor of the small ribosomal subunit (SSU) (Fig. 1a and b). Just as in the case of internal structure, the ability of the RNA replisome to bind single-stranded RNA has the advantage of high local concentration of interacting partners due to their tethering. The sequence-nonspecific binding of unstructured regions of nascent RNA to the replisome expanded the diversity of allowable replicating RNA structures.
The proposed model of evolution of random peptide synthesis. a In the RNA world, the replication was fulfilled by two different RNA molecules: the origin-recognizing subunit (Ori) and the catalytic riboreplisome (Rep). Ori became the ancestor of the small ribosomal subunit (SSU) RNA. Ori bound a tRNA-like structure (L-shaped) at the 3′ end of a structured genomic RNA template (dashed line) (Maizels and Weiner 1999) and recruited replisome to initiate replication. b SSU sliding along the nascent RNA (solid line) behind the replisome in the 5′ to 3′ direction prevented the annealing of the copy and template strands upstream of the replication fork. c Anchored at the nascent strand via tRNA, SSU stopped the expansion of the duplex formed by the copy and template strands annealing downstream. The environmental fluctuations could release the trapped replisome. d SSU peeled the copy strand off the template, applying tRNA as a molecular lever. The incoming tRNAs provided a driving force for forward translocation that actively rescued the trapped replisome. tRNAs were positioned adjacent to each other. e SSU employed tRNAs aminoacylated at 3′ end (circles). The replisome evolved into the large ribosomal subunit (LSU) directing transpeptidation coupled with forward translocation. Deacylated tRNA was removed, thus accelerating the turnover of tRNA. SSU and LSU formed the aminoacyl-tRNA-fueled RNA helicase. The noncoded formation of peptides resulted in useless stochastic byproducts that were released and discarded after the completion of replication
This SSU ancestor kept the copy and the template strands separate by sliding along unstructured stretches of nascent RNA in the 5′ to 3′ direction behind the growing RNA end. The hypothetical ancient SSU loaded onto the 5′ end of the nascent RNA or internally as soon as unstructured sequences became exposed. The proposed non-specific binding of 30S ribosomal subunits to the single-stranded standby sites for the purpose of translational initiation in E. coli (de Smit and van Duin 2003) is similar to the suggested action of its SSU ancestor. SSU did not load if the nascent RNA was highly structured. SSU then proceeded to slide along RNA until it encountered a structured region, at which point it dissociated. Alternatively, it could go all the way to the 3′ end if no stable structure was encountered. The nascent RNA was unlikely to fold behind SSU before the completion of the structural domain extension. In this case, replication would slow down or temporally stop, thus providing additional time for nascent RNA to form intramolecular structure. Replication could resume safely as soon as such a structure was formed. Alternatively, the loading of additional SSU(s) would expand the single-stranded RNA-binding capacity of the replisome.
tRNA as a Molecular Lever
If the copy and template strands annealed in front of the sliding SSU, the latter could slow down or stop the expansion of the duplex. SSU might apply a tRNA molecule to anchor on nascent RNA (Fig. 1c) in the same way as the modern SSU, with or without initiator tRNA, produces a toeprint by stopping reverse transcription downstream of a translation start (Moll et al. 2004; Wilson et al. 2000). Perhaps only a small fraction of replicating RNAs collapsed into the duplex in such a way. The anchoring of SSU to the replication intermediates through an external tRNA-like molecule could not be kinetically efficient without high concentration of available tRNAs. However, any molecular mechanism interfering with the expansion of the duplex could be beneficial and thus positively selected if the duplex-trapped replication complexes substantially contributed to the overall genetic instability (through sequence rearrangements and/or sequestration of essential RNA catalysts). Moreover, the template (and perhaps some RNA subunits of the replisome) could provide a high local tRNA concentration through the uncut tRNA-like structures at their 3′ ends (Fig. 1). Replication in such a trapped complex might resume only if environmental fluctuations melted the duplex.
I suggest that tRNA-like molecules had been originally engaged by the ancient SSU to release trapped replication complexes, like those shown on Fig. 1c. Applying tRNAs as molecular levers, ancient SSU acquired an ability to actively peel off the copy strand complexed in duplex with the template instead of passively relying on environmental fluctuations. The replisome locked at the annealed replication intermediates stopped the forward sliding of SSU along the copy strand. Next SSU bound to a tRNA molecule and directed it to the edge of the copy–template duplex, allowing the formation of the codon–anticodon-like basepairing with the nascent RNA (Fig. 1d). SSU could check several tRNAs before an appropriate anticodon was found. The simultaneous interaction of the nascent RNA with several consecutive tRNAs in a non-overlapping mode could make the interaction stronger and enhance the ability of SSU to release the replisome via the tRNA-like levers. The incoming tRNAs could provide a driving force for scanning (Cukras et al. 2003). No real coding or any other information processing would accompany such idle scanning. Thus, the term ‘anticodon’ refers only to the part of the tRNA-like molecules available for base pairing with the nascent RNA in the described process of scanning.
The SSU-mediated tRNA interaction with the nascent RNA should be relatively stable. Also, SSU should be able to scan any possible sequence of the nascent RNA with a minimal set of tRNAs. These simple limitations could lead to the domination of tRNAs having anticodons organized in triplets. Perhaps triplet anticodons had the ability to hold tRNA bound to the nascent RNA, which doublet anticodons lacked. Only 16 different tRNA-like molecules were required to interact with any sequence in the nascent RNA if their anticodons were organized in triplets and any pairing was acceptable at the first position of anticodons. Merely four tRNAs would be sufficient if the specific base pairing was limited with the second (central) base of anticodon (Wu et al. 2005). Such a scanning process could have been doubly degenerate from the beginning: one tRNA could interact with several related triplets, while different or overlapping sets of tRNA ancestors could have an ability to scan the same sequence. The SSU ancestor did not have to monitor the correct anticodon-mRNA pairing but reinforced any possible pairing. Obviously, there was no need to maintain the appearance of open reading frames so the exceptions with doublet or quadruplet anticodons were tolerated. In any case, the length of each scanning step was determined solely by the number of bases in the anticodon moiety of ancient tRNAs. Either the nonacylated or aminoacylated tRNA-like molecules or minihelixes containing only the anticodon portion of tRNA could fulfill the proposed function. If the ancient tRNA contained aminoacylated modules, the aminoacyl moiety did not participate in scanning and was available for interaction with any additional components. Nevertheless, tRNAs were positioned adjacent to each other, as was suggested for the primary function of the mini-ribosome (Brosius 2001). In contrast to a previously proposed theory (Orgel 1989), the current model hypothesizes that the external RNA (future mRNA)-directed association of several RNA adaptors (future tRNAs) preceded the untemplated formation of peptides.
A stalled replisome could allow SSU to discriminate between the duplex-trapped replication intermediates (Fig. 1c) and harmless structures formed by the nascent strand, thus initiating a tRNA-mediated scanning process. The genomic tag hypothesis postulated that the tRNA-like structures had been origins of replication located at 3′ ends of longer genomic RNAs in the RNA world (Maizels and Weiner 1999). Thus, an origin-recognizing subunit of an ancient RNA replisome was a possible ancestor of SSU, assuming that, in the RNA world, the origin recognition and replication activities were done by different molecules (similarly to the majority of modern replication systems). The origin-recognizing subunit had already been adapted to slide along the genomic RNA in search for an origin, bind tRNA-like structures, load the replisome, and perhaps locally melt the template (Fig. 1a). Once initiated, the proposed scanning with tRNAs could proceed as long as SSU remained in contact with the replisome. A structure formed in the nascent RNA might disrupt this contact. The termination of replication should result in folding of a tRNA-like structure at the 3′ end of the copy RNA (Maizels and Weiner 1999). The canning SSU could bind newly completed tRNA-like structures as a new origin of replication, thus switching from scanning to the next round of replication.
Origin of Peptide Synthesis Coupled with RNA Scanning
The suggested stepwise scanning process was able to rescue some collapsed replication complexes, but could reduce the overall rate of replication. The passive binding and dissociation of tRNAs would have been slow. I suggest that the synthesis of peptides evolved to accelerate the turnover of tRNAs in the energy-consuming elongation cycles, thus fueling the nascent RNA scanning. The increase in affinity to aminoacylated tRNAs together with the decrease in affinity to uncharged tRNAs stimulated tRNA turnover when accompanied by the repeating cycles of deacylation. The proposed acylation–deacylation cycle resembled the GTPase cycle of modern day G proteins, such as factors of protein synthesis. In addition to tRNA recycling, the energy of deacylation could be directly applied to the scanning process driving an aminoacyl-tRNA fueled RNA helicase (Fig. 1e).
Hydrolysis and transpeptidation constitute the two tRNA deacylation reactions performed by modern ribosomes. Transpeptidation allowed tight coupling of deacylation with RNA scanning (through association of several tRNAs in close proximity), whereas hydrolysis could occur with free tRNAs not participating in the scanning. Alternatively, transpeptidation was just a chemically preferable method of deacylation (Youngman et al. 2004). According to this scheme, transpeptidation fueled the scanning process by providing the additional (or the primary) driving force for translocation. Failure of the transpeptidation-based scanning to continue through the structured regions of nascent RNA (due to the inability to bind the next tRNA) resulted in hydrolysis, thus completing the scanning cycle. If acylated tRNAs were not available, the binding of the nonacylated tRNAs could also promote the release of peptides through hydrolysis.
This mode of polypeptide synthesis suggested the engagement of a transpeptidation-directing ribozyme. The aminoacylated ends of the ancient tRNAs arranged in close proximity to each other by the scanning SSU were substrates for this ancestor of large ribosomal RNA. Note that the aminoacylated end of tRNA did not directly participate in the scanning and was available for interaction with any auxiliary components. The process of scanning was coreplicational from the beginning so SSU always followed the RNA replisome. Thus the replisome, the ribozyme that had been the original partner of SSU RNA, was a logical ancestor of the large ribosomal subunit (LSU). The original replisome could acquire an additional peptide synthesis activity while retaining its primary catalytic function of RNA replication (Fig. 1e).
The noncoded formation of peptides (Orgel 1989) produced useless stochastic byproducts of replication. Perhaps such peptides were merely packaged in a generic fold and discarded. Random peptides could occasionally interfere with the normal scanning process by interacting with LSU or SSU RNA. Thus the first function dedicated specifically to polypeptides could be their disposal by a secretion-like process followed by the resumption of peptide synthesis. Only an RNA molecule was capable of fulfilling such a function in the RNA world. Other than that, the identity of amino acids in the produced peptides was irrelevant for the scanning. The original aminoacyl-tRNA synthetase probably had minimal (if any) ability to discriminate between individual amino acids. The amino acid diversity in the first polypeptides could be even greater than it is in the modern day proteins if the ancient aa-tRNA synthetase recognized only α-L configuration. The formation of peptides in a replication-assisting process did not require any selective advantage for rudimentary polypeptides, which was previously suggested as the driving force for evolution of the translation apparatus (Noller 2004; Orgel 1989).
Origin of the Genetic Code
The mobilization of aminoacylated tRNAs (cut from longer RNAs) for scanning of nascent transcripts did not eliminate the original function of tRNA-like structures as promoters of replication at 3′ ends of the genomic RNAs (Maizels and Weiner 1999). The recognition of amino acids as promoter elements corresponding to particular RNA species became possible only when tRNA-like structures were charged with specific amino acids. The specific aminoacylation probably began with the discrimination between groups of structurally similar amino acids (Orgel 1989). The anticodon loop of a tRNA-like structure was probably another specificity determinant of promoters. Thus, the selection for the genomic RNA recognition could lead to specificity in aminoacylation of the tRNA-like structure at the 3′ end of the genomic RNA. The subsequent excision of such tRNA-like structures from the mature functional RNAs created a pool of aminoacylated tRNA molecules with correspondence between the structure of the anticodon and the amino acid attached to the 3′ end (Maizels and Weiner 1999). This tRNA-specific aminoacylation was unrelated to the nascent RNA scanning and evolved independently. Note that in the modern translation apparatus, the information processing is clearly separated into two steps: aminoacyl-tRNA synthetases match the anticodon (as a part of tRNA) with the correct amino acid, whereas ribosomes fulfill the decoding of mRNA sequence.
The participation of the tRNA molecules that were aminoacylated in anticodon-dependent manner in the replication-coupled scanning produced peptide structures predetermined by the base sequence of the scanned transcript. Nevertheless, the synthesis of polypeptides remained a side reaction of the nascent RNA scanning until some polypeptides were positively selected for some function(s). The point in time when these polypeptides became advantageous and subsequently essential should be considered the beginning of the RNA-templated protein synthesis and the appearance of a new class of RNA, the mRNAs. The first proteins should have existed in the amount not exceeding the number of RNA templates since only one unique polypeptide could be formed per replication cycle of a repeat-free RNA even if the start of an open reading frame was strongly fixed. A reasonable function of the first proteins could have been the binding to ribozymes and the stabilization of active conformations similarly to the mode of action of the protein cofactor in bacterial RNase P (Buck et al. 2005). Perhaps the first RNAs associated with proteins were components of the replication machinery located close to the site of proteins synthesis. Therefore, the first proteins could be the ribosomal and/or replisomal ones as well as the cis-acting proteins binding their own templates (or complementary strands). Protein synthesis did not immediately diminish the original ribosomal role in maintaining newly synthesized RNA single-stranded. Ribosomes continued to scan nascent RNA during replication and produce nonfunctional polypeptides. Thus the expansion of the functions of ancient ribosomes (Fig. 1e) had to precede the conversion of ribosomes to structures specialized for protein synthesis (the switch of function). Acting as a mere supplement to the preexisting non-sequence-specific RNA scanning and polypeptide synthesizing machinery, the genetic code remained a relatively flexible component, “standing apart from the rest of translation” (Woese 2001).
Transition to Modern Ribosomes and Remnants of the Ancient Ribosome Functions
The conversion of polypeptide synthesis into the main ribosomal function (accompanied by the conversion of polypeptides to proteins) required some adjustments to the previously existing synthetic apparatus: (1) ribosomes gained the ability to scan mRNA for multiple rounds, thus partially uncoupling the translation and replication; (2) a pool of mRNAs had to be maintained even if these RNAs had no additional functions; (3) the requirement to maintain open reading frames eliminated tRNAs with anticodons shorter or longer than three bases; (4) initiation and termination codons mostly replaced the unstructured RNA regions as boundaries of open reading frames; (5) more stringent discrimination developed between cognate amino acids, thus increasing the accuracy of translation; (6) the genetic code became frozen.
The translational apparatus continued to evolve after the RNA world was replaced by the protein-RNA world. Perhaps the main step forward in this direction was the separation of the RNA and protein synthesis as can be observed in the modern day organisms. The appearance of DNA as a genetic substance created alternative methods for keeping the nascent RNA apart from its template. The conservative synthesis of RNA templated by double-stranded DNA made it easier to maintain the RNA product single-stranded due to the restoration of DNA helix behind the transcription bubble since the thermodynamic stability of DNA–DNA and RNA–DNA duplexes is comparable. However, the negative supercoiling generated upstream of the transcription bubbles made both prokaryotic and eukaryotic transcription prone to the formation of the R-loops (RNA/DNA heteroduplexes) (Gowrishankar and Harinarayanan 2004). Therefore, “the nascent RNA transcript generally has an inherent capacity to cause trouble” (Svejstrup 2003). Not surprisingly, the modern day transcription and translation are still coupled in bacteria. Ribosomes remain a positive transcriptional cofactor by preventing premature transcription termination known as nonsense polarity. Both translation and polarity protect bacterial transcription from the formation of R-loops by unstructured mRNAs (Gowrishankar and Harinarayanan 2004). The coupling of transcription and translation in bacteria is frequently defined as the beginning of the nascent mRNA translation before the completion of its synthesis. Perhaps the coupling is more intimate and the ribosome is a specific transcription elongation factor for coding sequences. This model explains the existence of a specific antitermination mechanism for the transcription of long noncoding ribosomal RNA precursors in bacteria (Quan et al. 2005). The participation of E. coli ribosomes in the regulation of gene expression via transcription attenuation (Yanofsky 2000) even more closely resembles original way of translation proposed here: the continuation of transcription requires the process of the leader peptide translation rather than the leader peptide itself.
The peptidyl transferase activity of modern ribosomes most likely provides the major energy source for the co-translational translocation of secretory proteins (Koch et al. 2003). However, the increasing accuracy of translation in the early organisms could no longer rely on the incoming aminoacyl-tRNA and/or transpeptidation as a driving force for translocation. G protein cofactors perhaps were originally recruited in order to regulate alternative decoding events (like the choice between continuing scanning and termination). Later, some G proteins became mandatory translation factors, promoting key steps of the elongation cycle (aminoacyl-tRNA binding and translocation) and coupling these steps with GTP hydrolysis. In E. coli, the ribosomal proteins S12 and S13 prevent the factor-independent translocation intrinsic to ribosome, which may indicate a relatively recent modification of the original scanning (Cukras et al. 2003). The modification of ribosomal proteins S12 and S13 re-enacts that ancient factor-independent translocation mechanism normally blocked in the bacterial translation apparatus (Cukras et al. 2003). The ribosomal helicase activity functions in absence of GTP, ATP, or added elongation factors, indicating that the energy released during the single-round translocation is sufficient to disrupt three base pairs of a mRNA duplex (Takyar et al. 2005).
Perhaps initiation and termination codons were originally used just for precise demarcation of open reading frame boundaries within the unstructured sequences of nascent transcripts. Later these codons were able to direct the multiple-round translation of mature mRNAs. The elongation factor GTPases allowed translation to progress through very structured mRNA regions. However, some structural elements in modern mRNAs continue to mark gene boundaries and/or participate in alternative decoding events. Programmed ribosome bypassing of a 50 nucleotide gap in decoding phage T4 gene 60 mRNA is stimulated by a stem-loop structure containing the take off (ribosome dissociation) site (Bucklin et al. 2005). Intriguingly, cricket paralysis virus (CrPV) and turnip yellow mosaic virus (TYMV), both containing plus-strand genomic RNA, retained a structure—rather than codon-based strategy for the translation start recognition of their structural protein precursor and polyprotein, respectively (Barends et al. 2003; Wilson et al. 2000). The majority of eukaryotic internal ribosome entry sites (IRES) utilize RNA structural elements for ribosome loading, but direct translation starts at the conventional initiation codons (Hellen and Sarnow 2001) and thus can be viewed as a transitional step from structure- to codon-determined initiation. The assignment of stop codons in modern life is also not completely fixed. SECIS, selenocysteine insertion sequence elements, direct the incorporation of selenocysteine at UGA stop codons. Some microbes also incorporate pyrrolysine at UAG stop codon (Namy et al. 2004). The original ribosome loading at the unstructured 5′ end of the nascent transcripts was preserved in the modern day leaderless mRNA found in all kingdoms of life (Marintchev and Wagner 2004; Moll et al. 2004). However, the more recent methods of ribosome binding to the internal bacterial Shine–Dalgarno sequence or to the 5′-cap in eukaryotes became more common. The evolution of the ribosome loading pathways for multiple-round translation apparently accompanied the divergence of the three domains of modern cellular life, Bacteria, Archeae, and Eukarya (Marintchev and Wagner 2004). Development of protein biosynthesis was probably a major factor that made cellular life possible, and the possession of ribosomes remains a universal difference between cells and viruses.
Discussion
The concept presented here explains and reevaluates some available biochemical data. Reasonable experimental predictions may be deduced from the model. The hypothesis suggests that the small and large ribosomal subunit (SSU and LSU) RNAs are the descendants of the origin-recognizing and catalytic moieties of an ancient riboreplisome, respectively. The ribosome lost its role as a replisome, but acquired the capacity for the multiple round translation of the mature mRNA instead. Perhaps the need for repeated translation was originally fulfilled by a replication-competent SSU/LSU pair (Fig. 1e). A reasonable way to switch the same ribosome from replication-assisting to multiple-round translation modes and vice versa could be the reversible binding of a structural analog mimicking the replication fork (a duplex of growing RNA-copy 3′ end with its template), which could induce a replication-associated conformation in nonreplicating ribosomes. If this model is correct, modern day ribosomes may retain a structure somewhere within LSU mimicking the ancient replication intermediates. If retained through evolution, such a structure was probably converted into an integral ribosomal element maintaining LSU in functional conformation.
This hypothesis suggests that tRNA-like structures were originally recruited to participate in translation for the purpose of safe reactivation of the arrested replication complexes (Fig. 1c), which alternatively could have stimulated other rescuing processes (such as recombination), causing genetic instability. It would be interesting to determine whether or not translating ribosomes contribute to the fidelity of RNA synthesis in modern life. It has been known for decades that the in vitro replication of phage Qβ genomic RNA with purified Qβ replicase leads to accumulation of deletions since shorter RNAs replicate faster and, in absence of external selection, overcompete genomic RNA (Mills et al. 1967). Perhaps such deletions are generated by the coreplicational recombination and the paused and/or arrested replication complexes are the substrates of these recombination events. The translating ribosomes in the coupled in vitro replication-translation system may release the trapped replication complexes, thereby preventing or slowing down the accumulation of deletions in the pool of replicating RNAs.
This hypothesis explains the extensive overlap between sets of factors participating in synthesis of RNA and proteins since both of these processes differentiated from the same original ribosome-assisted RNA replication apparatus. The translation elongation factors EF-Tu and EF-Ts are subunits of the phage Qβ RNA replicase (Blumenthal and Carmichael 1979). Proteins shared by the bacterial transcription and translation machineries are reviewed by Squires and Zaporojets (2000). The order of assembly of the translating ribosomes from the components of the translational apparatus, to some extent, recapitulates the sequence of evolutionary events that led to their development for translation-related functions. The current hypothesis is consistent with the theory that “only after proteins accompanied RNA and supplanted some of its functions did DNA become the carrier of genetic information” (Brosius 2001).
The hypothesis presented here suggests that the structural elements in mRNA played a broader role in ancient translation. If IRES is indeed a molecular remnant of the primordial structure-determined translation initiation signals, then the termination-driving RNA structural elements independent of (or additional to) termination codons may also survive in modern life forms. The present model of evolution explains why a nascent polypeptide is not the key factor maintaining stability of the elongation complex in the bacterial translation apparatus. The release of the polypeptide in response to stop codons is followed by recruitment of several factors to disassemble the post-termination complex (Peske et al. 2005; Zavialov et al. 2005). Without these factors, ribosomes continue scanning mRNA downstream stop codons and eventually resume polypeptide synthesis (Hirokawa et al. 2004). The suggested ancestry of the ribosome explains this ‘unscheduled translation’. The sliding of the elongating ribosomes over and beyond “hungry” codons and resumption of polypeptide synthesis downstream (Gallant and Lindsley 1998) also indicates that the process of scanning is more inherent to bacterial ribosomes than the external factor-guided production of functional proteins. The current model places the bacterial tmRNA, which combines the features of both tRNA and mRNA, not at the origin of translation (Brosius 2001) but rather at a later step of the evolutionary chronology, at the point when it became mandatory to punctuate open reading frames with stop codons. Nowadays, bacterial ribosomes have an intrinsic limit to the fidelity of protein synthesis, and some mutations that make ribosome more accurate do not have a selective advantage (Cochella and Green 2005; Ogle and Ramakrishnan 2005). The present hypothesis suggests that the enhanced fidelity of ribosomes may disfavor the coupling of transcription and translation in addition to slowing down the overall rate of protein synthesis and suppressing some mutations (Cochella and Green 2005; Ogle and Ramakrishnan 2005).
This hypothesis suggests that amino acids had passed through a strong selection for compatibility with the transpeptidation-driven RNA scanning process before a subsequent selection as structural and functional modules of proteins. The chemical diversity of amino acids (and their combinations) could indeed be a challenge for the ancient RNA-scanning ribosomes. Perhaps the set of amino acids available for the incorporation into first proteins was already restricted. Directed toward the replication of RNA, original ribosomes disposed polypeptides as useless byproducts. Such a disposal seems to be a possible precursor of the co-translational secretion process in modern day organisms. This model explains the participation of RNA-containing signal recognition particles in co-translational secretion in all three domains of cellular life (Koch et al. 2003). The hypothesis proposes that the ancient ribosomes scanned mostly (if not exclusively) unstructured regions of the nascent RNAs, which could be interrupted by structured elements. Such discontinuous coding regions could evolve into both the prokaryotic polycistronic mRNAs or the eukaryotic intron-containing pre-mRNAs. The coding regions could be assembled from unstructured sequences originally distributed among unlinked RNA molecules by a process similar to trans-splicing. Therefore, exon shuffling by spliceosomes in eukaryotes might evolve through the adaptation of some features of mobile RNA elements for creating the original coding sequences, in contrast to the model that spliceosomes developed to rescue formerly continuous genes after the invasion by and subsequent degeneration of mobile group II introns (Martin and Koonin 2006).
The hypothesis suggests that ancient ribosomes played dual roles: as protein synthesis machineries repeatedly translating mRNAs and as cofactors of RNA replication scanning any nascent RNA, with the accompanying synthesis of mostly nonfunctional polypeptides. Such a double function may explain the differentiation of eukaryotic nucleus and cytoplasm as separate compartments where ribosomes fulfill either one or the other task, depending on the presence of the auxiliary cofactors. The intriguing possibility of coupled transcription and translation within the nuclei of the mammalian cells remains controversial (Iborra et al. 2001). This can be a remnant of the RNA synthesis involving the obligatory assistance of ribosomes that continue to scan modern day mRNA. If such a relict of the original role of ribosomes still operates (perhaps for regulatory purposes), it can produce nonfunctional polypeptides directed for degradation (similar to bacterial leader peptides). The cotranscriptional peptide formation in nuclei may have different initiation and termination signals as well as open reading frames (assuming that peptides are not involved in regulatory functions of coupled transcription and translation). A process of idle mRNA scanning by ribosomes (similar to that postulated by the current hypothesis) was suggested for a quality-control mechanism of mRNA known as nonsense-mediated decay (Wilkinson and Shyu 2002). Even in the cytoplasm, the initial (“pioneer”) round of mRNA translation is fulfilled with a different set of protein cofactors and detects premature stop codons (Ishigaki et al. 2001). Alternatively, the evolution of RNA polymerase II for transcription of mRNAs in eukaryotes could replace the original ribosomal cofactors in the translation-coupled transcription of coding RNAs.
Conclusion
In summary, this paper proposes the following model for the origin of protein synthesis:
-
1.
An ancestor of small ribosomal subunit (SSU) evolved as an auxiliary subunit of the RNA replisome to keep apart the copy and template RNA strands.
-
2.
tRNAs were recruited as molecular levers to break down the undesired duplexes formed by the replication intermediates.
-
3.
Peptide synthesis by the ancestor of the large ribosomal subunit (LSU) evolved as a motor, fueling the RNA helicase and accelerating the tRNA recycling.
-
4.
The independently-developed tRNA-specific aminoacylation was superimposed on the preexisting peptide-synthesizing machinery, resulting in the template-based production of proteins.
The keystone idea is the evolution of the protein synthesis apparatus from a replicational tool that pealed the nascent RNA off its template. The ribosome originated as an aminoacyl tRNA-fueled RNA helicase. The characteristic features of translation evolved through the following steps:
-
Sliding of a SSU ancestor in 5′ to 3′ direction along single-stranded regions of the nascent RNA.
-
Coreplicational stepwise scanning of the nascent RNA by the SSU ancestor using the tRNA-like levers.
-
The formation of random polypeptides as byproducts of tRNA turnover during scanning.
-
The emergence of the genetic code and the appearance of encoded polypeptides.
-
The conversion of the RNA-scanning apparatus into the protein-synthesizing machinery.
References
Axelrod VD, Brown E, Priano C, Mills DR (1991) Coliphage Q beta RNA replication: RNA catalytic for single-strand release. Virology 184:595–608
Barends S, Bink HH, van den Worm SH, Pleij CW, Kraal B (2003) Entrapping ribosomes for viral translation: tRNA mimicry as a molecular Trojan horse. Cell 112:123–129
Blumenthal T, Carmichael GG (1979) RNA replication: function and structure of Qbeta-replicase. Annu Rev Biochem 48:525–548
Brosius J (2001) tRNAs in the spotlight during protein biosynthesis. Trends Biochem Sci 26:653–656
Buck AH, Dalby AB, Poole AW, Kazantsev AV, Pace NR (2005) Protein activation of a ribozyme: the role of bacterial RNase P protein. EMBO J 24:3360–3368
Bucklin DJ, Wills NM, Gesteland RF, Atkins JF (2005) P-site pairing subtleties revealed by the effects of different tRNAs on programmed translational bypassing where anticodon re-pairing to mRNA is separated from dissociation. J Mol Biol 345:39–49
Campbell JH (1991) An RNA replisome as the ancestor of the ribosome. J Mol Evol 32:3–5
Cochella L, Green R (2005) Fidelity in protein synthesis. Curr Biol 15:R536–R540
Cukras AR, Southworth DR, Brunelle JL, Culver GM, Green R (2003) Ribosomal proteins S12 and S13 function as control elements for translocation of the mRNA:tRNA complex. Mol Cell 12:321–328
de Smit MH, van Duin J (2003) Translational standby sites: how ribosomes may deal with the rapid folding kinetics of mRNA. J Mol Biol 331:737–743
Gallant JA, Lindsley D (1998) Ribosomes can slide over and beyond “hungry” codons, resuming protein chain elongation many nucleotides downstream. Proc Natl Acad Sci USA 95:13771–13776
Gilbert W (1986) The RNA world. Nature 319:618
Gowrishankar J, Harinarayanan R (2004) Why is transcription coupled to translation in bacteria? Mol Microbiol 54:598–603
Hellen CU, Sarnow P (2001) Internal ribosome entry sites in eukaryotic mRNA molecules. Genes Dev 15:1593–1612
Hirokawa G, Inokuchi H, Kaji H, Igarashi K, Kaji A (2004) In vivo effect of inactivation of ribosome recycling factor—fate of ribosomes after unscheduled translation downstream of open reading frame. Mol Microbiol 54:1011–1021
Iborra FJ, Jackson DA, Cook PR (2001) Coupled transcription and translation within nuclei of mammalian cells. Science 293:1139–1142
Ishigaki Y, Li X, Serin G, Maquat LE (2001) Evidence for a pioneer round of mRNA translation: mRNAs subject to nonsense-mediated decay in mammalian cells are bound by CBP80 and CBP20. Cell 106:607–617
Joyce GF (2002) The antiquity of RNA-based evolution. Nature 418:214–221
Koch HG, Moser M, Muller M (2003) Signal recognition particle-dependent protein targeting, universal to all kingdoms of life. Rev Physiol Biochem Pharmacol 146:55–94
Kramer FR, Mills DR (1981) Secondary structure formation during RNA synthesis. Nucleic Acids Res 9:5109–5124
Maizels N, Weiner AM (1999) The genomic tag hypothesis: what molecular fossils tell us about the evolution of tRNA. In: Gesteland RF, Cech TR, Atkins JF (eds) The RNA world, 2nd edn. Cold Spring Harbor Laboratory Press, New York, pp 79–111
Marintchev A, Wagner G (2004) Translation initiation: structures, mechanisms and evolution. Q Rev Biophys 37:197–284
Martin W, Koonin EV (2006) Introns and the origin of nucleus-cytosol compartmentalization. Nature 440:41–45
Mills DR, Peterson RL, Spiegelman S (1967) An extracellular Darwinian experiment with a self-duplicating nucleic acid molecule. Proc Natl Acad Sci USA 58:217–224
Moll I, Hirokawa G, Kiel MC, Kaji A, Blasi U (2004) Translation initiation with 70S ribosomes: an alternative pathway for leaderless mRNAs. Nucleic Acids Res 32:3354–3363
Morozov IY, Ugarov VI, Chetverin AB, Spirin AS (1993) Synergism in replication and translation of messenger RNA in a cell-free system. Proc Natl Acad Sci USA 90:9325–9329
Namy O, Rousset JP, Napthine S, Brierley I (2004) Reprogrammed genetic decoding in cellular gene expression. Mol Cell 13:157–168
Noller HF (2004) The driving force for molecular evolution of translation. RNA 10:1833–1837
Ogle JM, Ramakrishnan V (2005) Structural insights into translational fidelity. Annu Rev Biochem 74:129–177
Orgel LE (1989) The origin of polynucleotide-directed protein synthesis. J Mol Evol 29:465–474
Orgel LE (2003) Some consequences of the RNA world hypothesis. Orig Life Evol Biosph 33:211–218
Peske F, Rodnina MV, Wintermeyer W (2005) Sequence of steps in ribosome recycling as defined by kinetic analysis. Mol Cell 18:403–412
Poot RA, Tsareva NV, Boni IV, van Duin J (1997) RNA folding kinetics regulates translation of phage MS2 maturation gene. Proc Natl Acad Sci USA 94:10110–10115
Priano C, Kramer FR, Mills DR (1987) Evolution of the RNA coliphages: the role of secondary structures during RNA replication. Cold Spring Harbor Symp Quant Biol 52:321–330
Quan S, Zhang N, French S, Squires CL (2005) Transcriptional polarity in rRNA operons of Escherichia coli nusA and nusB mutant strains. J Bacteriol 187:1632–1638
Sledz CA, Williams BR (2004) RNA interference and double-stranded-RNA-activated pathways. Biochem Soc Trans 32:952–956
Squires CL, Zaporojets D (2000) Proteins shared by the transcription and translation machines. Annu Rev Microbiol 54:775–798
Svejstrup J (2003) Keeping RNA and DNA apart during transcription. Mol Cell 12:538–539
Takyar S, Hickerson RP, Noller HF (2005) mRNA helicase activity of the ribosome. Cell 120:49–58
Wilkinson MF, Shyu AB (2002) RNA surveillance by nuclear scanning? Nat Cell Biol 4:E144–E147
Wilson JE, Pestova TV, Hellen CU, Sarnow P (2000) Initiation of protein synthesis from the A site of the ribosome. Cell 102:511–520
Woese CR (2001) Translation: in retrospect and prospect. RNA 7:1055–1067
Wu HL, Bagby S, van den Elsen JM (2005) Evolution of the genetic triplet code via two types of doublet codons. J Mol Evol 61:54–64
Yanofsky C (2000) Transcription attenuation: once viewed as a novel regulatory strategy. J Bacteriol 182:1–8
Youngman EM, Brunelle JL, Kochaniak AB, Green R (2004) The active site of the ribosome is composed of two layers of conserved nucleotides with distinct roles in peptide bond formation and peptide release. Cell 117:589–599
Zavialov AV, Hauryliuk VV, Ehrenberg M (2005) Splitting of the posttermination ribosome into subunits by the concerted action of RRF and EF-G. Mol Cell 18:675–686
Acknowledgements
I am greatly thankful to my daughter Anastasiya Yakhnina for carefully editing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yakhnin, A.V. A Model for the Origin of Protein Synthesis as Coreplicational Scanning of Nascent RNA. Orig Life Evol Biosph 37, 523–536 (2007). https://doi.org/10.1007/s11084-007-9108-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-007-9108-z