Abstract
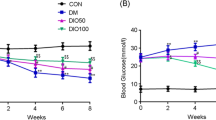
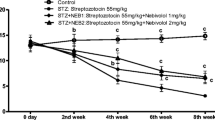
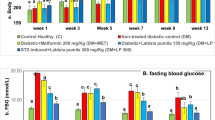
Diabetes is a multifunctional chronic disease that affects both the central and/or peripheral nervous systems. This study assessed whether nicotinamide (NAm) or conjugate of nicotinic acid with gamma-aminobutyric acid (N-GABA) could be potential neuroprotective agents against type 1 diabetes (T1D)-induced nervous system impairments in rats. After six weeks of T1D, induced by streptozotocin, nonlinear male Wistar rats were treated for two weeks with NAm (100 mg/kg, i. p.) or N-GABA (55 mg/kg, i. p.). Expression levels of myelin basic protein (MBP) were analyzed by immunoblotting. Polyol pathway parameters of the sciatic nerves were assessed spectrophotometrically, and their structure was examined histologically. NAm had no effect on blood glucose or body weight in T1D, while N-GABA reduced glucose by 1.5-fold. N-GABA also increased MBP expression by 1.48-fold, enhancing neuronal myelination, while NAm showed no such effect. Activation of the polyol pathway was observed in the T1D sciatic nerves. Both compounds decreased sorbitol content and aldose reductase activity, thereby alleviating changes similar to primary degeneration in the sciatic nerves and preventing peripheral neuropathy development. These results demonstrate that NAm and, more notably, N-GABA may exert neuroprotective effects against T1D-induced nervous system impairments by increasing MBP expression levels, improving myelination processes in the brain, inhibiting the polyol pathway, and partially restoring morphometric parameters in the sciatic nerves. This suggests their potential therapeutic efficacy as promising agents for the prevention of T1D-induced nervous system alterations.




Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
Abbreviations
- BBB:
-
Blood-Brain Barrier
- BNB:
-
Blood-Nerve Barrier
- CNS:
-
Central Nervous System
- DPN:
-
Diabetic Peripheral Neuropathy
- GABA:
-
Gamma-Aminobutyric Acid
- GFAP:
-
Glial Fibrillary Acidic Protein
- i.p.:
-
Intraperitoneal
- MBP:
-
Myelin Basic Protein
- NAm:
-
Nicotinamide
- NAD+ :
-
Nicotinamide Adenine Dinucleotide
- NADP+ :
-
Nicotinamide Adenine Dinucleotide Phosphate
- NADH:
-
Nicotinamide Adenine Dinucleotide Hydrogen
- NADPH:
-
Nicotinamide Adenine Dinucleotide Phosphate Hydrogen
- N-GABA:
-
Nicotinoyl-GABA
- Nf-L:
-
Neurofilament Light Chain
- PARP-1:
-
Poly-ADP-Ribose Polymerase-1
- PNS:
-
Peripheral Nervous System
- SD:
-
Standard Deviation
- SIRT1:
-
NAD-dependent protein deacetylase sirtuin-1
- SIRT2:
-
NAD-dependent protein deacetylase sirtuin-2
- SEM:
-
Scanning Electron Microscopy
- STZ:
-
Streptozotocin
- T1D:
-
Type 1 Diabetes
- T2D:
-
Type 2 Diabetes
References
Cole JB, Florez JC (2020) Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol 16(7):377–390. https://doi.org/10.1038/s41581-020-0278-5
Popoviciu MS, Kaka N, Sethi Y, Patel N, Chopra H, Cavalu S (2023) Type 1 diabetes Mellitus and Autoimmune diseases: a critical review of the Association and the application of Personalized Medicine. J Pers Med 13(3):422. https://doi.org/10.3390/jpm13030422
King A, Bowe J (2016) Animal models for diabetes: understanding the pathogenesis and finding new treatments. Biochem Pharmacol 99:1–10. https://doi.org/10.1016/j.bcp.2015.08.108
Mezza T, Cinti F, Cefalo CMA, Pontecorvi A, Kulkarni RN, Giaccari A (2019) β-Cell fate in human insulin resistance and type 2 diabetes: a perspective on Islet plasticity. Diabetes 68(6):1121–1129. https://doi.org/10.2337/db18-0856
Braffett BH, Gubitosi-Klug RA, Albers JW, Feldman EL, Martin CL, White NH et al (2020) Risk factors for Diabetic Peripheral Neuropathy and Cardiovascular Autonomic Neuropathy in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and complications (DCCT/EDIC) study. Diabetes 69(5):1000–1010. https://doi.org/10.2337/db19-1046
Biessels GJ, Whitmer RA (2020) Cognitive dysfunction in diabetes: how to implement emerging guidelines. Diabetologia 63:3–9. https://doi.org/10.1007/s00125-019-04977-9
Elafros MA, Andersen H, Bennett DL, Savelieff MG, Viswanathan V, Callaghan BC, Feldman EL (2022) Towards prevention of diabetic peripheral neuropathy: clinical presentation, pathogenesis, and new treatments. Lancet Neurol 21(10):922–936. https://doi.org/10.1016/S1474-4422(22)00188-0
Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, Sosenko JM, Ziegler D (2017) Diabetic Neuropathy: A position Statement by the American Diabetes Association. Diabetes Care 40(1):136–154. https://doi.org/10.2337/dc16-2042
Rastogi A, Goyal G, Kesavan R et al (2020) Long term outcomes after incident diabetic foot ulcer: Multicenter large cohort prospective study (EDI-FOCUS investigators) epidemiology of diabetic foot complications study: epidemiology of diabetic foot complications study. Diabetes Res Clin Pract 162:108113. https://doi.org/10.1016/j.diabres.2020.108113
Kuchmerovska T, Shymanskyy I, Donchenko G, Kuchmerovskyy M, Pakirbaieva L, Klimenko A (2004) Poly(ADP-ribosyl)ation enhancement in brain cell nuclei is associated with diabetic neuropathy. J Diabetes Complications 18(4):198–204. https://doi.org/10.1016/S1056-8727(03)00039-4
Guzyk M, Dyakun K, Yanytska O, Pryvrotska I, Krynytska I, Pishel’ I, Kuchmerovska T (2017) Inhibitors of poly(adp-ribose)polymerase-1 as agents providing correction of brain dysfunctions induced by experimental diabetes. Neurophysiology 49(3):183–193. https://doi.org/10.1007/s11062-017-9672-4
Guzyk M, Tykhonenko T, Dyakun K, Yanitska L, Pryvrotska I, Kuchmerovska T (2019) Altered sirtuins 1 and 2 expression in the brain of rats induced by experimental diabetes and the ways of its correction. Ukr Biochem J 91(1):21–29. https://doi.org/10.15407/ubj91.01.021
Fricker RA, Green EL, Jenkins SI, Griffin SM (2018) The influence of Nicotinamide on Health and Disease in the Central Nervous System. Int J Tryptophan Res 11:1–11. https://doi.org/10.1177/1178646918776658
Napolitano T, Avolio F, Vieira A, Ben-Othman N, Courtney M, Gjernes E, Hadzic B, Druelle N, Navarro Sanz S, Silvano S, Mansouri A, Collombat P (2017) GABA signaling stimulates α-cell-mediated β-like cell neogenesis. Commun Integr Biol 10(3):e1300215. https://doi.org/10.1080/19420889.2017.1300215
Lorenz-Guertin JM, Jacob TC (2018) GABA type a receptor trafficking and the architecture of synaptic inhibition. Dev Neurobiol 78(3):238–270. https://doi.org/10.1002/dneu.22536
Trikash I, Gumenyuk V, Kuchmerovska T (2015) Diabetes-Induced impairments of the exocytosis process and the Effect of Gabapentin: the link with cholesterol level in neuronal plasma membranes. Neurochem Res 40:723–732. https://doi.org/10.1007/s11064-015-1520-6
Drel V, Pacher P, Stavniichuk R, Xu W, Zhang J, Kuchmerovska T, Slusher B, Obrosova I (2011) Poly(ADP-ribose)polymerase inhibition counteracts renal hypertrophy and multiple manifestations of peripheral neuropathy in diabetic akita mice. Int J Mol Med 28(4):629–635. https://doi.org/10.3892/ijmm.2011.709
Guzyk M, Tykhomyrov A, Nedzvetsky V, Prischepa I, Grinenko T, Yanitska L, Kuchmerovska T (2016) Poly(ADP-ribose) polymerase-1 (parp-1) inhibitors reduce reactive gliosis and improve angiostatin levels in retina of diabetic rats. Neurochem Res 41(10):2526–2537. https://doi.org/10.1007/s11064-016-1964-3
Tykhonenko Т, Guzyk M, Tykhomyrov A, Korsa V, Yanitska L, Kuchmerovska T (2022) Modulatory effects of vitamin B3 and its derivative on the levels of apoptotic and vascular regulators and cytoskeletal proteins in diabetic rat brain as signs of neuroprotection. Biochimica et Biophysica Acta (BBA) -. Gen Subj 1866(11):130207. https://doi.org/10.1016/j.bbagen.2022.130207
Eftekharpour E, Fernyhough P (2022) Oxidative stress and Mitochondrial Dysfunction Associated with Peripheral Neuropathy in Type 1 diabetes. Antioxid Redox Signal 37(7–9):578–596. https://doi.org/10.1089/ars.2021.0152
Zhang P, Li T, Wu X, Nice EC, Huang C, Zhang Y (2020) Oxidative stress and diabetes: antioxidative strategies. Front Med 14(5):583–600. https://doi.org/10.1007/s11684-019-0729-1
Kaestner KH, Powers AC, Naji A, Consortium H, Atkinson MA (2019) NIH Initiative to improve understanding of the pancreas, islet, and autoimmunity in type 1 diabetes: the human pancreas analysis program (HPAP). Diabetes 68(7):1394–1402. https://doi.org/10.2337/db19-0058
Akbari M, Hassan-Zadeh V (2018) Hyperglycemia affects the expression of inflammatory genes in Peripheral Blood mononuclear cells of patients with type 2 diabetes. Immunol Invest 47(7):654–665. https://doi.org/10.1080/08820139.2018.1480031
Traiffort E, Kassoussi A, Zahaf A, Laouarem Y (2020) Astrocytes and microglia as major players of myelin production in normal and pathological conditions. Front Cell Neurosci 14:79. https://doi.org/10.3389/fncel.2020.00079
Kıray H, Lindsay SL, Hosseinzadeh S, Barnett SC (2016) The multifaceted role of astrocytes in the regulation of myelination. Exp Neurol 283(Pt B 541–549. https://doi.org/10.1016/j.expneurol.2016.03.009
Camargo N, Goudriaan A, van Deijk AF, Otte WM, Brouwers JF, Lodder H, Gutmann DH, Nave KA, Dijkhuizen RM, Mansvelder HD, Chrast R, Smit AB, Verheijen MHG (2017) Oligodendroglial myelination requires astrocyte-derived lipids. PLoS Biol 15(5):e1002605. https://doi.org/10.1371/journal.pbio.1002605
Li J, Guan R, Pan L (2023) Mechanism of Schwann cells in diabetic peripheral neuropathy: a review. Medicine 102(1):e32653. https://doi.org/10.1097/MD.0000000000032653
Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL et al (2019) Diabetic neuropathy. Nat Rev Dis Primers 5(1):41. https://doi.org/10.1038/s41572-019-0092-1
Zhu J, Hu Z, Luo Y, Liu Y, Luo W, Du X, Luo Z, Hu J, Peng S (2024) Diabetic peripheral neuropathy: pathogenetic mechanisms and treatment. Front Endocrinol 14:1265372. https://doi.org/10.3389/fendo.2023.1265372
Cherian CM, Reeves HR, De Silva D, Tsao S, Marshall KE, Rideout EJ (2024) Consideration of sex as a biological variable in diabetes research across twenty years. Biol Sex Differ 15(19). https://doi.org/10.1186/s13293-024-00595-2
Zucker I, Beery A (2010) Males still dominate animal studies. Nature 465(690). https://doi.org/10.1038/465690a
Stoscheck C (1990) Quantitation of protein. Methods Enzymol 182:50–68. https://doi.org/10.1016/0076-6879(90)82008-p
Bergmeyer H (1965) Methods of enzymatic analysis, 2nd edn. Verlag Chemie, New York and London
Gumenyuk A, Rybalko S, Ryzha A, Savosko S, Labudzynskyi D, Levchuk N, Chaikovsky Y (2018) Nerve regeneration in conditions of HSV-Infection and an antiviral drug influence. Anat Rec 301(10):1734–1744. https://doi.org/10.1002/ar.23848
Singh M, Kapoor A, Bhatnagar A (2021) Physiological and pathological roles of Aldose Reductase. Metabolites 11(10):655. https://doi.org/10.3390/metabo11100655
Chato-Astrain J, García-García ÓD, Campos F, Sánchez-Porras D, Carriel V (2021) Basic nerve histology and histological analyses following peripheral nerve repair and regeneration. Peripheral Nerve Tissue Engineering and Regeneration Reference Series in Biomedical Engineering. Springer, Cham, pp 1–37. https://doi.org/10.1007/978-3-030-06217-0_14-1
Gasperi V, Sibilano M, Savini I, Catani MV (2019) Niacin in the central nervous system: an update of biological aspects and clinical applications. Int J Mol Sci 20(4):974. https://doi.org/10.3390/ijms20040974
Fricker RA, Green EL, Jenkins SI, Griffin SM (2018) The influence of Nicotinamide on Health and Disease in the Central Nervous System. Int J Tryptophan Res 11:1178646918776658. https://doi.org/10.1177/1178646918776658
Fricker RA, Green EL, Jenkins SI, Griffin SM (2018) The influence of Nicotinamide on Health and Disease in the Central Nervous System. Int J Tryptophan Res 11. https://doi.org/10.1177/1178646918776658
Rai SN, Singh P, Steinbusch HWM, Vamanu E, Ashraf G, Singh MP (2021) The role of vitamins in neurodegenerative disease: an update. Biomedicines 9(10):1284. https://doi.org/10.3390/biomedicines9101284
Takasawa S (2022) CD38-Cyclic ADP-Ribose Signal System in Physiology, Biochemistry, and pathophysiology. Int J Mol Sci 23(8):4306. https://doi.org/10.3390/ijms23084306
Sears SM, Hewett SJ (2021) Influence of glutamate and GABA transport on brain excitatory/inhibitory balance. Exp Biol Med (Maywood) 246(9):1069–1083. https://doi.org/10.1177/1535370221989263
Korol SV, Jin Z, Jin Y, Bhandage AK, Tengholm A, Gandasi NR, Barg S, Espes D, Carlsson PO, Laver D, Birnir B (2018) Functional characterization of native, High-Affinity GABAA receptors in human pancreatic β cells. EBioMedicine 30:273–282. https://doi.org/10.1016/j.ebiom.2018.03.014
Ben-Othman N, Vieira A, Courtney M, Record F, Gjernes E, Avolio F, Hadzic B, Druelle N, Napolitano T, Navarro-Sanz S, Silvano S, Al-Hasani K, Pfeifer A, Lacas-Gervais S, Leuckx G, Marroquí L, Thévenet J, Madsen OD, Eizirik DL, Heimberg H, Kerr-Conte J, Pattou F, Mansouri A, Collombat P (2017) Long-term GABA administration induces alpha cell-mediated Beta-like cell Neogenesis. Cell 168(1–2):73–85E11. https://doi.org/10.1016/j.cell.2016.11.002
Bhandage AK, Jin Z, Korol SV, Shen Q, Pei Y, Deng Q, Espes D, Carlsson PO, Kamali-Moghaddam M, Birni B (2018) GABA regulates release of inflammatory cytokines from peripheral blood mononuclear cells and CD4 + T cells and is immunosuppressive in type 1 diabetes. EBioMedicine 30:283–294. https://doi.org/10.1016/j.ebiom.2018.03.019
Dulin WE, Wyse BM (1969) Studies on the ability of compounds to block the diabetogenic activity of streptozotocin. Diabetes 18(7):459–466. https://doi.org/10.2337/diab.18.7.459
Wang KL, Tao M, Wei TJ, Wei R (2021) Pancreatic β cell regeneration induced by clinical and preclinical agents. World J Stem Cells 13(1):64–77. https://doi.org/10.4252/wjsc.v13.i1.64
Kister A, Kister I (2023) Overview of myelin, major myelin lipids, and myelin-associated proteins. Front Chem 10:1041961. https://doi.org/10.3389/fchem.2022.1041961
Köhler S, Winkler U, Hirrlinger J (2021) Heterogeneity of astrocytes in Grey and White Matter. Neurochem Res 46(1):3–14. https://doi.org/10.1007/s11064-019-02926-x
Tobore TO (2021) Oxidative/Nitroxidative stress and multiple sclerosis. J Mol Neurosci 71(3):506–514. https://doi.org/10.1007/s12031-020-01672-y
Ngo DH, Vo TS (2019) An updated review on Pharmaceutical properties of Gamma-Aminobutyric Acid. Molecules 24(15):2678. https://doi.org/10.3390/molecules24152678
Reyes-Haro D, Cisneros-Mejorado A, Arellano RO (2021) Therapeutic potential of GABAergic Signaling in myelin plasticity and repair. Front Cell Dev Biol 9:662191. https://doi.org/10.3389/fcell.2021.662191
Yan LJ (2018) Redox imbalance stress in diabetes mellitus: role of the polyol pathway. Anim Model Exp Med 1(1):7–13. https://doi.org/10.1002/ame2.12001
Chang KC, Shieh B, Petrash JM (2019) Role of aldose reductase in diabetes-induced retinal microglia activation. Chem Biol Interact 302:46–52. https://doi.org/10.1016/j.cbi.2019.01.020
Hao W, Tashiro S, Hasegawa T et al (2015) Hyperglycemia promotes Schwann Cell de-differentiation and de-myelination via Sorbitol Accumulation and Igf1 protein down-regulation. J Biol Chem 290(28):17106–17115. https://doi.org/10.1074/jbc.M114.631291
Inceoglu B, Bettaieb A, Trindade da Silva CA, Lee KS, Haj FG, Hammock BD (2015) Endoplasmic reticulum stress in the peripheral nervous system is a significant driver of neuropathic pain. Proc Natl Acad Sci 112(29):9082–9087. https://doi.org/10.1073/pnas.1510137112
Rachana K, Manu M, Advirao G (2016) Insulin influenced expression of myelin proteins in diabetic peripheral neuropathy. Neurosci Lett 629:110–115. https://doi.org/10.1016/j.neulet.2016.06.067
Lirk P, Verhamme C, Boeckh R, Stevens MF, ten Hoope W, Gerner P, Blumenthal S, de Girolami U, van Schaik IN, Hollmann MW, Picardi S (2015) Effects of early and late diabetic neuropathy on sciatic nerve block duration and neurotoxicity in zucker diabetic fatty rats. Br J Anaesth 114(2):319–326. https://doi.org/10.1093/bja/aeu270
Stavniichuk R, Drel VR, Shevalye H, Maksimchyk Y, Kuchmerovska TM, Nadler JL, Obrosova IG (2011) Baicalein alleviates diabetic peripheral neuropathy through inhibition of oxidative-nitrosative stress and p38 MAPK activation. Exp Neurol 230(1):106–113. https://doi.org/10.1016/j.expneurol.2011.04.002
Palova D, Turic Csokova N, Markova K, Kontsekova E, Kovacech B, Zilkova M (2021) The engagement of microglia in tau-targeted immunotherapy in Alzheimer’s disease. Gen Physiol Biophys 40(6):463–478. https://doi.org/10.4149/gpb_2021029. PMID: 34897021
Nelson AR, Sweeney MD, Sagare AP, Zlokovic BV (2016) Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer’s disease. Biochim Biophys Acta 1862(5):887–900. https://doi.org/10.1016/j.bbadis.2015.12.016
Sweeney MD, Sagare AP, Zlokovic BV (2018) Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 14:133–150. https://doi.org/10.1038/nrneurol.2017.188
Richner M, Ferreira N, Dudele A, Jensen TS, Vaegter CB, Goncalves NP (2018) Functional and structural changes of the blood-nerve-barrier in Diabetic Neuropathy. Front Neurosci 12:1038. https://doi.org/10.3389/fnins.2018.01038
Kuchmerovska T, Shymanskyy I, Chlopicki S, Klimenko A (2010) L-methylnicotinamide (MNA) in prevention of diabetes-associated brain disorders. Neurochem Int 56(2):221–228. https://doi.org/10.1016/j.neuint.2009.10.004
Kuchmerovska TM, Dyakun KO, Guzyk MM, Yanytska LV, Pryvrotska IB (2019) Effects of a combined mitochondria-targeted treatment on the state of mitochondria and synaptic membranes from the brains of diabetic rats. Neurophysiology 51(4):234–247. https://doi.org/10.1007/s11062-019-09816-6
Funding
This work was funded by the budget program of National Academy of Sciences of Ukraine.
Author information
Authors and Affiliations
Contributions
T.K. wrote the main manuscript text and did conceptualization, methodology, reviewing & editing. T.T. prepared Fig. 1 and did investigation, methodology, visualization and data curation. S.S. prepared Fig. 2 and did methodology, visualization and data curation. L.Y. and I.P. did investigation, visualization and data curation. All authors helped to critically revise the intellectual content of the manuscript and approved the final submission.
Corresponding author
Ethics declarations
Ethical Approval
All animal experiments were carried out in accordance with the Directive 2010/63/ EU and approved by the Animal Care and Use Committee of Palladin Institute of Biochemistry, NAS of Ukraine.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kuchmerovska, T., Tykhonenko, T., Yanitska, L. et al. Nicotinamide and Nicotinoyl-Gamma-Aminobutyric Acid as Neuroprotective Agents Against Type 1 Diabetes-Induced Nervous System Impairments in Rats. Neurochem Res 50, 1 (2025). https://doi.org/10.1007/s11064-024-04257-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11064-024-04257-y