Abstract
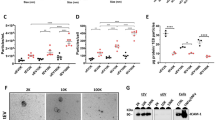
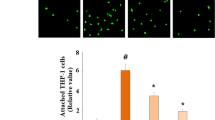
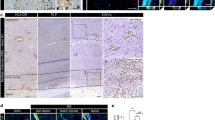
The blood–brain barrier (BBB) normally bars peripheral T lymphocytes from entering the cerebrum. Interestingly, activated T cells exist as infiltrates in the brains of Alzheimer’s disease (AD) patients, but little is known about the mechanisms involved. In this study, we observed significantly higher MHC class I expression in rat brain endothelial cells compared with controls following the induction of experimental AD models. An in vitro BBB model, which was constructed with human brain microvascular endothelial cells, was established to study the mechanisms underlying the transendothelial migration of T cells. Using in vitro studies, we demonstrated that secretion of TNF-α from Aβ1–42-treated BV2 microglia contributes to the elevated expression of MHC class I on the brain microvessel endothelium. Transmigration assays and adhesion assays confirmed that the upregulation of MHC class I molecules was associated with T cell transendothelial migration. MHC class I knock-down in HBMECs significantly attenuated the migratory and adhesive capability of the T cells. Interestingly, a TNF-α neutralizing antibody effectively blocked the transendothelial migration of T cells triggered by treatment with the supernatant from Aβ1–42-treated BV2 microglia. We propose that microglia-derived TNF-α upregulates MHC class I molecule expression on brain endothelial cells, which represents a mechanism of T cell migration into the brain. This study may provide a new insight into the potential pathomechanism of Alzheimer’s disease.






Similar content being viewed by others
References
Ross CA, Poirier MA (2004) Protein aggregation and neurodegenerative disease. Nat Med 10(Suppl):S10–S17. doi:10.1038/nm1066
Selkoe DJ (2001) Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev 81(2):741–766
Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K (2006) Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci USA 103(30):11172–11177
Blennow K, de Leon MJ, Zetterberg H (2006) Alzheimer’s disease. Lancet 368(9533):387–403
Selkoe DJ (2002) Alzheimer’s disease is a synaptic failure. Science 298(5594):789–791. doi:10.1126/science.1074069
Hoozemans JJ, Rozemuller AJ, Veerhuis R, Eikelenboom P (2001) Immunological aspects of alzheimer’s disease: therapeutic implications. BioDrugs 15(5):325–337
Eikelenboom P, Bate C, Van Gool WA, Hoozemans JJ, Rozemuller JM, Veerhuis R, Williams A (2002) Neuroinflammation in Alzheimer’s disease and prion disease. Glia 40(2):232–239. doi:10.1002/glia.10146
Rogers J, Strohmeyer R, Kovelowski CJ, Li R (2002) Microglia and inflammatory mechanisms in the clearance of amyloid beta peptide. Glia 40(2):260–269. doi:10.1002/glia.10153
Weiner HL, Frenkel D (2006) Immunology and immunotherapy of Alzheimer’s disease. Nat Rev Immunol 6(5):404–416
Grasso P (2002) Essentials of pathology for toxicologists. Informa HealthCare, London
Alberts B (2002) Molecular biology of the cell, 4th edn. Garland Science, New York
Johnston H, Boutin H, Allan SM (2011) Assessing the contribution of inflammation in models of Alzheimer’s disease. Biochem Soc Trans 39(4):886–890. doi:10.1042/BST0390886
Sulger J, Dumais-Huber C, Zerfass R, Henn FA, Aldenhoff JB (1999) The calcium response of human T lymphocytes is decreased in aging but increased in Alzheimer’s dementia. Biol Psychiatry 45(6):737–742
Tan J, Town T, Abdullah L, Wu Y, Placzek A, Small B, Kroeger J, Crawford F, Richards D, Mullan M (2002) CD45 isoform alteration in CD4+ T cells as a potential diagnostic marker of Alzheimer’s disease. J Neuroimmunol 132(1–2):164–172
Monsonego A, Zota V, Karni A, Krieger JI, Bar-Or A, Bitan G, Budson AE, Sperling R, Selkoe DJ, Weiner HL (2003) Increased T cell reactivity to amyloid beta protein in older humans and patients with Alzheimer disease. J Clin Invest 112(3):415–422. doi:10.1172/JCI18104
Town T, Tan J, Flavell RA, Mullan M (2005) T-cells in Alzheimer’s disease. Neuromolecular Med 7(3):255–264
Schindowski K, Eckert A, Peters J, Gorriz C, Schramm U, Weinandi T, Maurer K, Frolich L, Muller WE (2007) Increased T-cell reactivity and elevated levels of CD8 + memory T-cells in Alzheimer’s disease-patients and T-cell hyporeactivity in an Alzheimer’s disease-mouse model: implications for immunotherapy. Neuromolecular Med 9(4):340–354. doi:10.1007/s12017-007-8015-9
Miscia S, Ciccocioppo F, Lanuti P, Velluto L, Bascelli A, Pierdomenico L, Genovesi D, Di Siena A, Santavenere E, Gambi F, Ausili-Cefaro G, Grimley PM, Marchisio M, Gambi D (2009) Abeta(1–42) stimulated T cells express P-PKC-delta and P-PKC-zeta in Alzheimer disease. Neurobiol Aging 30(3):394–406
Ciccocioppo R, Rossi M, Pesce I, Ricci G, Millimaggi D, Maurano F, Corazza GR (2008) Effects of gliadin stimulation on bone marrow-derived dendritic cells from HLA-DQ8 transgenic MICE. Dig Liver Dis 40(12):927–935. doi:10.1016/j.dld.2008.05.005
Magaki S, Yellon SM, Mueller C, Kirsch WM (2008) Immunophenotypes in the circulation of patients with mild cognitive impairment. J Psychiatr Res 42(3):240–246
Itagaki S, McGeer PL, Akiyama H (1988) Presence of T-cytotoxic suppressor and leucocyte common antigen positive cells in Alzheimer’s disease brain tissue. Neurosci Lett 91(3):259–264
Rogers J, Luber-Narod J, Styren SD, Civin WH (1988) Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer’s disease. Neurobiol Aging 9(4):339–349
Togo T, Akiyama H, Iseki E, Kondo H, Ikeda K, Kato M, Oda T, Tsuchiya K, Kosaka K (2002) Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J Neuroimmunol 124(1–2):83–92
Monsonego A, Imitola J, Zota V, Oida T, Weiner HL (2003) Microglia-mediated nitric oxide cytotoxicity of T cells following amyloid beta-peptide presentation to Th1 cells. J Immunol 171(5):2216–2224
Monsonego A, Weiner HL (2003) Immunotherapeutic approaches to Alzheimer’s disease. Science 302(5646):834–838. doi:10.1126/science.1088469
Seguin R, Biernacki K, Prat A, Wosik K, Kim HJ, Blain M, McCrea E, Bar-Or A, Antel JP (2003) Differential effects of Th1 and Th2 lymphocyte supernatants on human microglia. Glia 42(1):36–45. doi:10.1002/glia.10201
Townsend KP, Town T, Mori T, Lue LF, Shytle D, Sanberg PR, Morgan D, Fernandez F, Flavell RA, Tan J (2005) CD40 signaling regulates innate and adaptive activation of microglia in response to amyloid beta-peptide. Eur J Immunol 35(3):901–910. doi:10.1002/eji.200425585
Liu YJ, Guo DW, Tian L, Shang DS, Zhao WD, Li B, Fang WG, Zhu L, Chen YH (2010) Peripheral T cells derived from Alzheimer’s disease patients overexpress CXCR2 contributing to its transendothelial migration, which is microglial TNF-alpha-dependent. Neurobiol Aging 31(2):175–188. doi:10.1016/j.neurobiolaging.2008.03.024
Man SM, Ma YR, Shang DS, Zhao WD, Li B, Guo DW, Fang WG, Zhu L, Chen YH (2007) Peripheral T cells overexpress MIP-1alpha to enhance its transendothelial migration in Alzheimer’s disease. Neurobiol Aging 28(4):485–496
Stins MF, Gilles F, Kim KS (1997) Selective expression of adhesion molecules on human brain microvascular endothelial cells. J Neuroimmunol 76(1–2):81–90
Lai JP, Douglas SD, Zhao M, Ho WZ (1999) Quantification of substance P mRNA in human mononuclear phagocytes and lymphocytes using a mimic-based RT-PCR. J Immunol Methods 230(1–2):149–157
Hudson LC, Bragg DC, Tompkins MB, Meeker RB (2005) Astrocytes and microglia differentially regulate trafficking of lymphocyte subsets across brain endothelial cells. Brain Res 1058(1–2):148–160
Muthusamy A, Achur RN, Valiyaveettil M, Botti JJ, Taylor DW, Leke RF, Gowda DC (2007) Chondroitin sulfate proteoglycan but not hyaluronic acid is the receptor for the adherence of Plasmodium falciparum-infected erythrocytes in human placenta, and infected red blood cell adherence up-regulates the receptor expression. Am J Pathol 170(6):1989–2000
Walker DG, Lue LF, Beach TG (2001) Gene expression profiling of amyloid beta peptide-stimulated human post-mortem brain microglia. Neurobiol Aging 22(6):957–966
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297(5580):353–356. doi:10.1126/science.1072994
Franciosi S, Ryu JK, Choi HB, Radov L, Kim SU, McLarnon JG (2006) Broad-spectrum effects of 4-aminopyridine to modulate amyloid beta1–42-induced cell signaling and functional responses in human microglia. J Neurosci 26(45):11652–11664
Weitz TM, Town T (2012) Microglia in Alzheimer’s disease: it’s all about context. Int J Alzheimers Dis 2012:314185. doi:10.1155/2012/314185
Grab DJ, Nikolskaia O, Kim YV, Lonsdale-Eccles JD, Ito S, Hara T, Fukuma T, Nyarko E, Kim KJ, Stins MF, Delannoy MJ, Rodgers J, Kim KS (2004) African trypanosome interactions with an in vitro model of the human blood–brain barrier. J Parasitol 90(5):970–979. doi:10.1645/GE-287R
Huang SH, Chen YH, Fu Q, Stins M, Wang Y, Wass C, Kim KS (1999) Identification and characterization of an Escherichia coli invasion gene locus, ibeB, required for penetration of brain microvascular endothelial cells. Infect Immun 67(5):2103–2109
Engelhardt B (2006) Molecular mechanisms involved in T cell migration across the blood–brain barrier. J Neural Transm 113(4):477–485. doi:10.1007/s00702-005-0409-y
Brabb T, von Dassow P, Ordonez N, Schnabel B, Duke B, Goverman J (2000) In situ tolerance within the central nervous system as a mechanism for preventing autoimmunity. J Exp Med 192(6):871–880
Ransohoff RM, Kivisakk P, Kidd G (2003) Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol 3(7):569–581. doi:10.1038/nri1130
Marelli-Berg FM, Frasca L, Weng L, Lombardi G, Lechler RI (1999) Antigen recognition influences transendothelial migration of CD4+ T cells. J Immunol 162(2):696–703
Pober JS, Kluger MS, Schechner JS (2001) Human endothelial cell presentation of antigen and the homing of memory/effector T cells to skin. Ann N Y Acad Sci 941:12–25
Tooyama I, Kimura H, Akiyama H, McGeer PL (1990) Reactive microglia express class I and class II major histocompatibility complex antigens in Alzheimer’s disease. Brain Res 523(2):273–280
Male DK, Pryce G, Hughes CC (1987) Antigen presentation in brain: MHC induction on brain endothelium and astrocytes compared. Immunology 60(3):453–459
Male D, Pryce G (1989) Induction of Ia molecules on brain endothelium is related to susceptibility to experimental allergic encephalomyelitis. J Neuroimmunol 21(1):87–90
Hirschberg H (1981) Presentation of viral antigens by human vascular endothelial cells in vitro. Hum Immunol 2(3):235–246
Burger DR, Ford D, Vetto RM, Hamblin A, Goldstein A, Hubbard M, Dumonde DC (1981) Endothelial cell presentation of antigen to human T cells. Hum Immunol 3(3):209–230
Hirschberg H, Hirschberg T, Jaffe E, Thornsby E (1981) Antigen-presenting properties of human vascular endothelial cells: inhibition by anti-HLA-DR antisera. Scand J Immunol 14(5):545–553
Murray AG, Libby P, Pober JS (1995) Human vascular smooth muscle cells poorly co-stimulate and actively inhibit allogeneic CD4+ T cell proliferation in vitro. J Immunol 154(1):151–161
Biedermann BC, Pober JS (1999) Human vascular endothelial cells favor clonal expansion of unusual alloreactive CTL. J Immunol 162(12):7022–7030
Candore G, Balistreri CR, Colonna-Romano G, Lio D, Caruso C (2004) Major histocompatibility complex and sporadic Alzheimer’s disease: a critical reappraisal. Exp Gerontol 39(4):645–652. doi:10.1016/j.exger.2003.10.027
Colton CA, Mott RT, Sharpe H, Xu Q, Van Nostrand WE, Vitek MP (2006) Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J Neuroinflammation 3:27
Michelucci A, Heurtaux T, Grandbarbe L, Morga E, Heuschling P (2009) Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: effects of oligomeric and fibrillar amyloid-beta. J Neuroimmunol 210(1–2):3–12. doi:10.1016/j.jneuroim.2009.02.003
Maezawa I, Zimin PI, Wulff H, Jin LW (2011) Amyloid-beta protein oligomer at low nanomolar concentrations activates microglia and induces microglial neurotoxicity. J Biol Chem 286(5):3693–3706. doi:10.1074/jbc.M110.135244
Colton C, Wilcock DM (2010) Assessing activation states in microglia. CNS Neurol Disord: Drug Targets 9(2):174–191
Lee S, Varvel NH, Konerth ME, Xu G, Cardona AE, Ransohoff RM, Lamb BT (2010) CX3CR1 deficiency alters microglial activation and reduces beta-amyloid deposition in two Alzheimer’s disease mouse models. Am J Pathol 177(5):2549–2562. doi:10.2353/ajpath.2010.100265
Mori T, Koyama N, Arendash GW, Horikoshi-Sakuraba Y, Tan J, Town T (2010) Overexpression of human S100B exacerbates cerebral amyloidosis and gliosis in the Tg2576 mouse model of Alzheimer’s disease. Glia 58(3):300–314. doi:10.1002/glia.20924
Sundaram JR, Chan ES, Poore CP, Pareek TK, Cheong WF, Shui G, Tang N, Low CM, Wenk MR, Kesavapany S (2012) Cdk5/p25-induced cytosolic PLA2-mediated lysophosphatidylcholine production regulates neuroinflammation and triggers neurodegeneration. J Neurosci 32(3):1020–1034. doi:10.1523/JNEUROSCI.5177-11.2012
Zhu Y, Hou H, Rezai-Zadeh K, Giunta B, Ruscin A, Gemma C, Jin J, Dragicevic N, Bradshaw P, Rasool S, Glabe CG, Ehrhart J, Bickford P, Mori T, Obregon D, Town T, Tan J (2011) CD45 deficiency drives amyloid-beta peptide oligomers and neuronal loss in Alzheimer’s disease mice. J Neurosci 31(4):1355–1365. doi:10.1523/JNEUROSCI.3268-10.2011
Colton CA (2009) Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol 4(4):399–418. doi:10.1007/s11481-009-9164-4
Wheway J, Obeid S, Couraud PO, Combes V, Grau GE (2013) The brain microvascular endothelium supports T cell proliferation and has potential for alloantigen presentation. PLoS ONE 8(1):e52586. doi:10.1371/journal.pone.0052586
Acknowledgments
We are grateful to Drs. Monique Stins and Kwang Sik Kim (Department of Pediatrics, Johns Hopkins University School of Medicine) for providing the HBMECs. The authors would like to thank Dong-Xin Liu for technical assistance in image processing. The authors have no financial conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yi-Ming Yang and De-Shu Shang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, YM., Shang, DS., Zhao, WD. et al. Microglial TNF-α-Dependent Elevation of MHC Class I Expression on Brain Endothelium Induced by Amyloid-Beta Promotes T Cell Transendothelial Migration. Neurochem Res 38, 2295–2304 (2013). https://doi.org/10.1007/s11064-013-1138-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-013-1138-5